Method for diagnosing hepatitis e virus infection and kit
A technology for hepatitis E virus and hepatitis E, which is applied in the field of immunological diagnosis and medicine, and can solve the problems of ELISA detection method, such as lower sensitivity, easy false positive or limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1. Detection of hepatitis E virus antigen (HEV-Ag) in urine
[0157] With the informed consent of the patient, urine samples and serum samples at the same time point were collected from a 35-year-old patient with chronic hepatitis E (Baoding Infectious Disease Hospital, Hebei Province). Urine samples and serum samples were then tested for HEV antigen (ORF2 antigen), nucleic acid, IgM and IgG antibodies using commercially available kits (see above) according to the manufacturer's instructions. The levels of alanine aminotransferase and aspartate transaminase in serum samples were detected using conventional kits (Japanese Wako Pure Chemical Industries, Ltd., catalog number: KH695). Before testing, serum samples and urine samples were stored in a -80°C refrigerator. The test results are shown in Table 1.
[0158] Table 1: Test results of urine and serum samples from a patient with chronic hepatitis E
[0159]
[0160]
[0161] Note: ALT, alanine aminotran...
Embodiment 2
[0171]Example 2. Relationship between HEV antigens in urine and HEV antigens in other types of samples
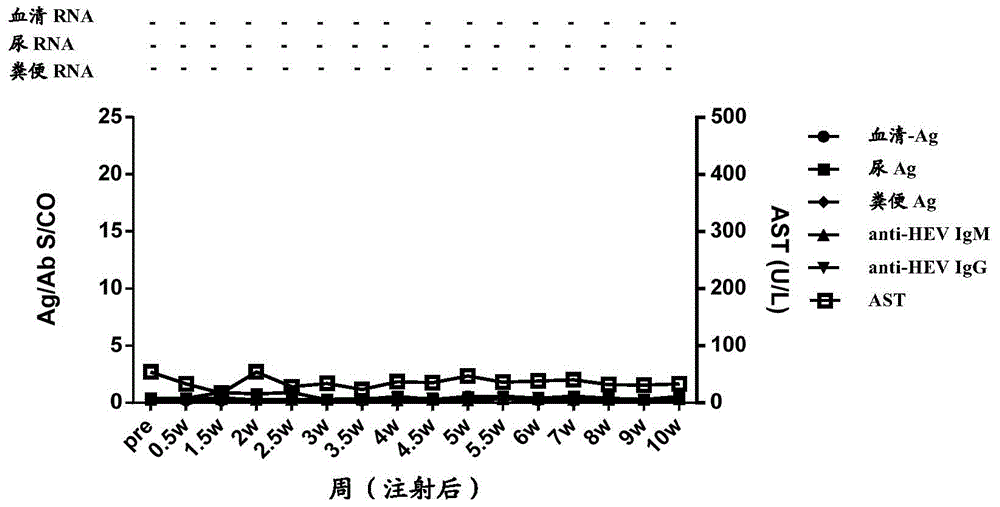
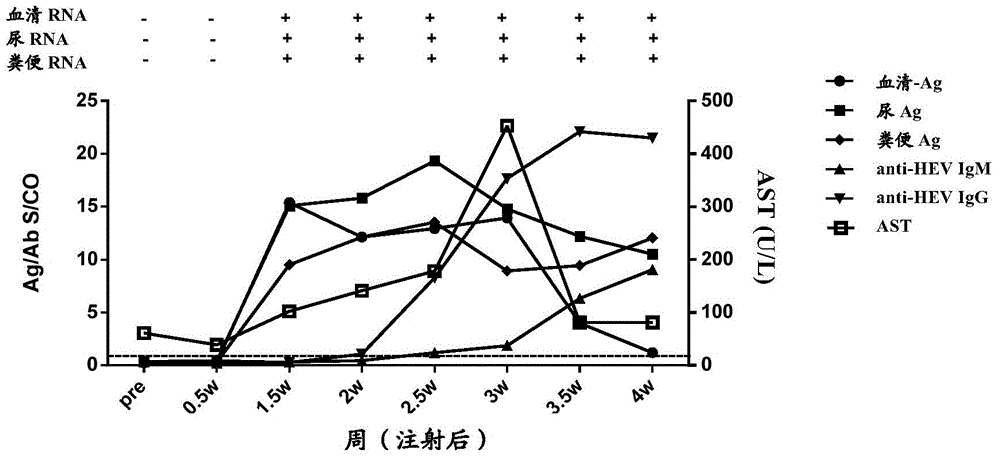
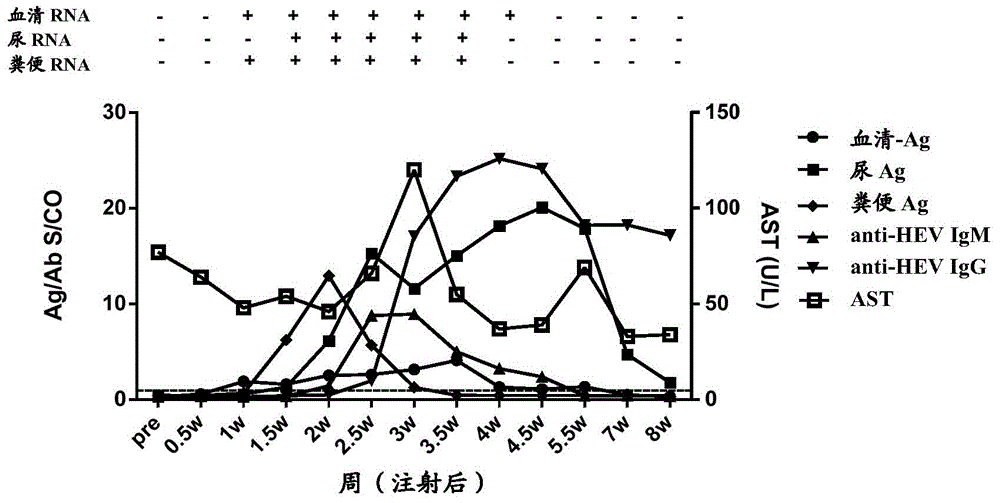
[0172] In order to study the time point when HEV antigens in urine appeared after the body was infected with HEV, and the relationship between HEV antigens in urine and HEV antigens in other types of samples, a primate model of HEV infection in cynomolgus monkeys was established. A total of 5 cynomolgus monkeys (Beijing Xierxin Institute of Biological Resources) were used. Among them, the cynomolgus monkey numbered 13C07004 (hereinafter referred to as 7004) was attacked with human type 4 HEV (strain WQ); the number was 13C07008 ( Cynomolgus monkeys hereinafter referred to as 7008) were attacked with human-source type 1 HEV (strain W2-1); cynomolgus monkeys numbered 13C08007 (hereinafter referred to as 8007) were attacked with W2-4; numbered 13C01063 (hereinafter referred to as 1063) The cynomolgus monkeys were challenged with the urine of 7004 cynomolgus monkeys infected wi...
Embodiment 3
[0199] Example 3. Detection of urine samples treated with pretreatment reagents
[0200] Three HEV-Ag positive urine samples were taken and diluted 1:1 with pretreatment reagents containing phosphate, calf serum and TritonX-100 to obtain pretreated urine samples. Parallel detection of HEV-Ag was performed on pretreated urine samples and unpretreated raw urine samples. The test results are shown in Table 4.
[0201] Table 4: HEV-Ag Detection Results of Pretreated and Unpretreated Urine Samples
[0202]
[0203] The detection result of table 4 shows: (1) after processing 1# sample with pretreatment reagent, the sensitivity of the inventive method improves, and detection result changes into true positive by false negative; (2) after processing 2# with pretreatment reagent and After the 3# sample, the detection result (HEV-Ag detection value) did not change significantly; however, considering the dilution effect of the pretreatment reagent on the sample, the HEV-Ag detection ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com