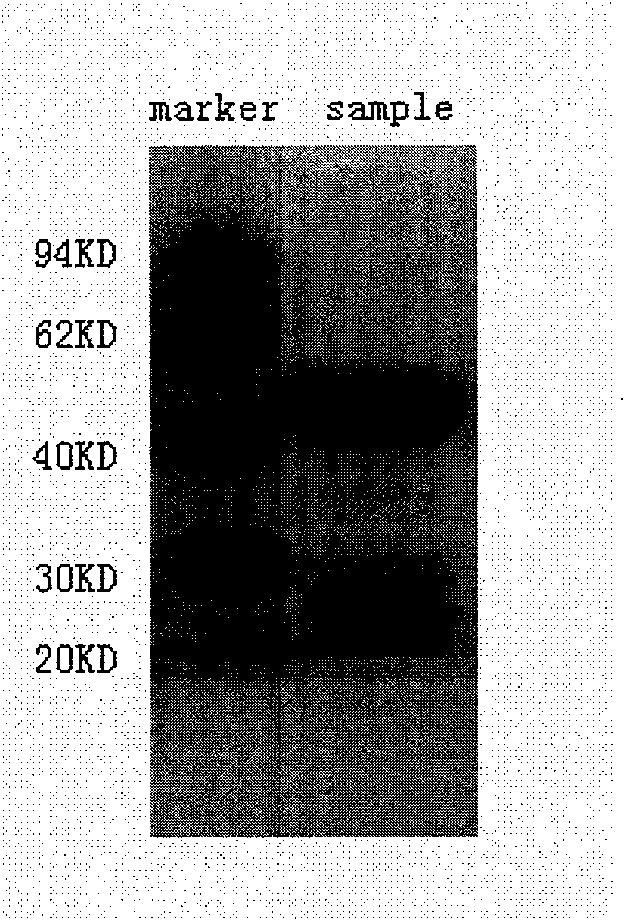
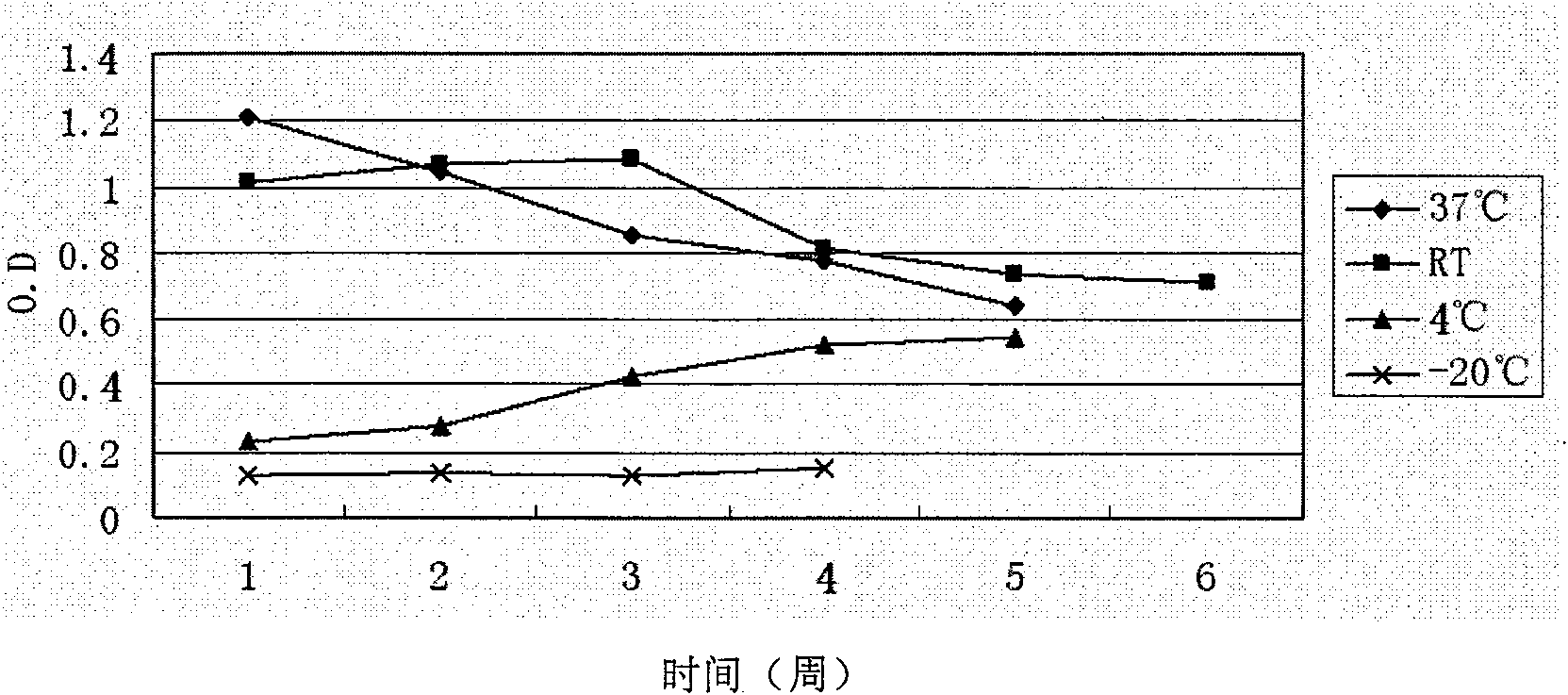
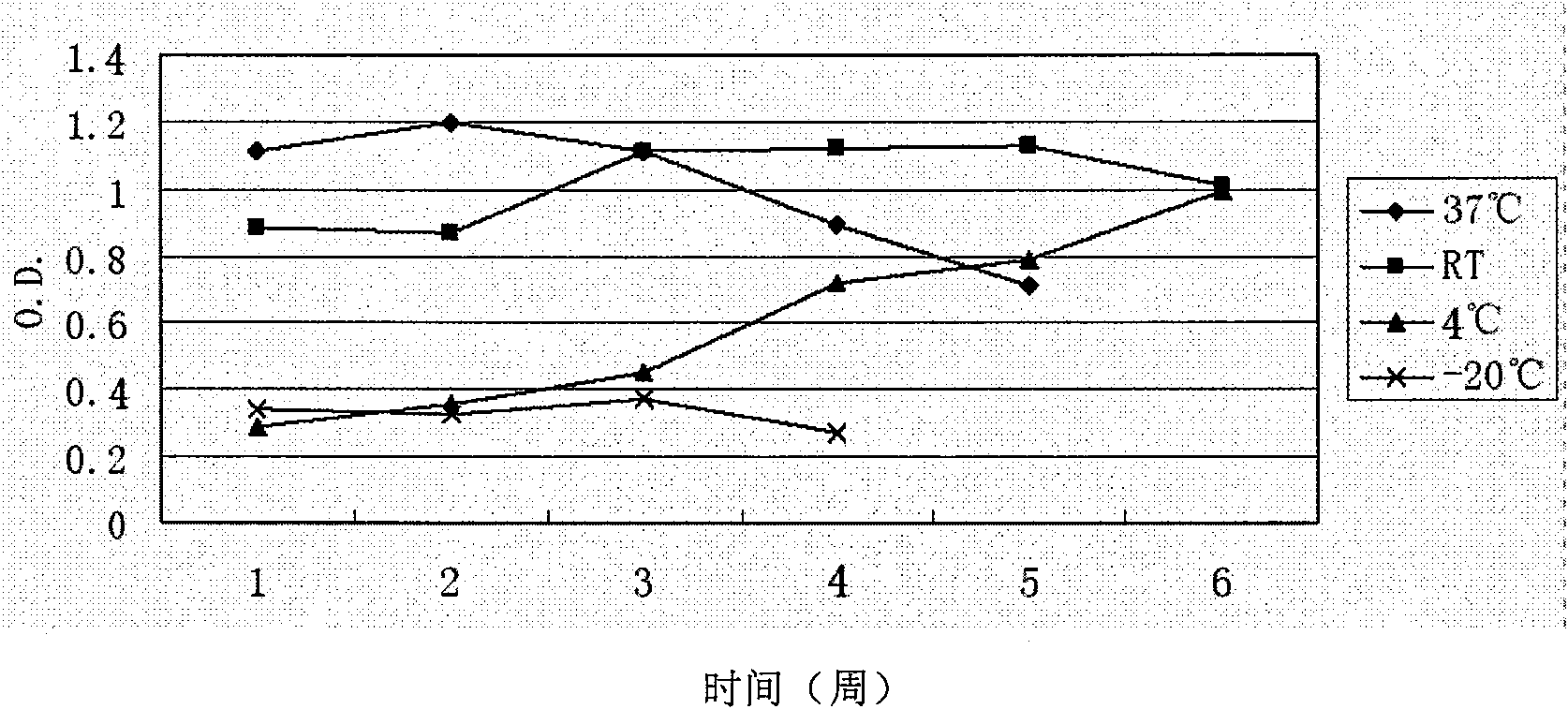
Preparation method of E hepatitis rabbit-human chimeric antibody quality control substance
A technology of chimeric antibody and hepatitis E, which is applied in the field of clinical laboratory science and biology, can solve the problems of prolonging the preparation cycle of quality control substances, low antibody affinity and complexity, etc., and achieve the effect of easy high titer mass acquisition and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Preparation of purification reagents:
[0055] (1) Neutralizing solution: 1M Tris-HCl, pH 9.0: Dissolve 12.11g Tris in 80ml of distilled water, add concentrated hydrochloric acid to adjust the pH to 9.0 (about 7.5ml), add water to make up to 100ml.
[0056] (2) Binding buffer: 20mM sodium phosphate, pH7.0, dissolve 3.8012g sodium phosphate in 500ml water, adjust the pH to 7.0 (about 1.5ml) with concentrated hydrochloric acid.
[0057] (3) Elution buffer: 0.58% (v; v) glacial acetic acid NaCl (0.15M) solution, NaCl (0.15M) solution: 4.383g NaCl was dissolved in 500ml of water, and then 1.45ml of glacial acetic acid was added.
[0058] Protein SDS-polyacrylamide gel electrophoresis reagent preparation (see Molecular Cloning Volume 2 P1716)
[0059] (1) 1×Tris-glycine electrophoresis buffer: can be prepared with 5× storage solution, add 15.1g Tris base and 94g glycine in 900ml deionized water, add 50ml 10% SDS (m / V), make up to 1000ml with deionized water.
[0060] (2) C...
Embodiment 1
[0072] Embodiment 1: the preparation of rabbit anti-human HEV-IgG
[0073] 1. Materials
[0074] 1. New Zealand white rabbits, healthy males, weighing about 2.5kg, from Beijing Xingfu Animal Farm.
[0075] 2. Antigen: NE2, batch number: 20060829, content 1.817mg / ml, Beijing Wantai Biological Pharmaceutical Co., Ltd.
[0076] 3. Hepatitis E IgM virus antibody diagnostic kit, Beijing Wantai Biopharmaceutical Co., Ltd.
[0077] 2. Method
[0078] 1. Serum Preparation
[0079] Blood was collected from the ear vein, and 1-2ml of blood was collected in a 1.5ml eppendorf centrifuge tube for future reference. Let the rabbit blood stand at room temperature for several hours, transfer the serum to a new centrifuge tube, centrifuge at 5000rpm for 10 minutes to collect the serum, put the serum in a 1.5ml tube, and store it at -70°C.
[0080] 2. Antigen Preparation
[0081] Take 10 μl of the original antigen solution and dilute it to 1 ml with 0.9% saline for injection (the content i...
Embodiment 2
[0145] Example 2: Antibody Crosslinking
[0146] 1. Method
[0147] 1. Rabbit anti-HEV-IgG cross-linked with human IgM
[0148] (1) Take (about 180μl) and (about 427μl) (1mgIgG and 1mgIgM) dialyzed with MES cross-linking buffer
[0149] (2) Equilibrate EDC to room temperature
[0150] (3) Mix the above two proteins after dialysis. (1mgIgG and 1mgIgM)
[0151] (4) Take 2.5 mg of EDC, dissolve it in 250 μl of water, and add 100 μl to the reaction system.
[0152] (5) React at room temperature for 2 hours.
[0153] (6) Dialyze with PBS overnight (1000ml) Dialyze overnight, during which the liquid is changed three times, and the interval is placed at 4 degrees, and stirred.
[0154] 2. Determination of chimeric antibody titer by ELISA
[0155] (1) Add 100 μl sample diluent to each well.
[0156] (2) Add negative control, EDC cross-linked product and positive control 10 μl in sequence (both make two wells).
[0157] (3) Incubate at 37°C for 30 minutes, wash plate 5 times.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com