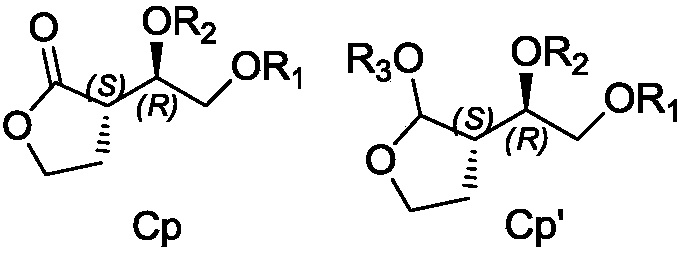
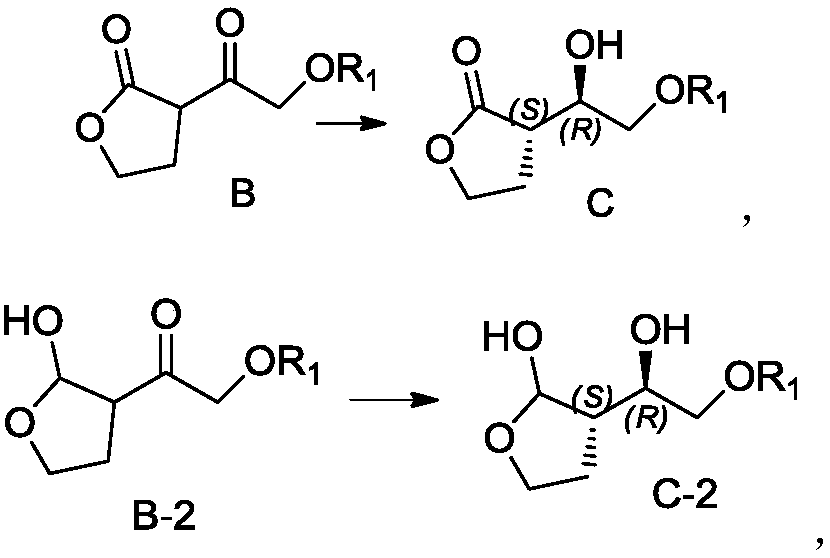
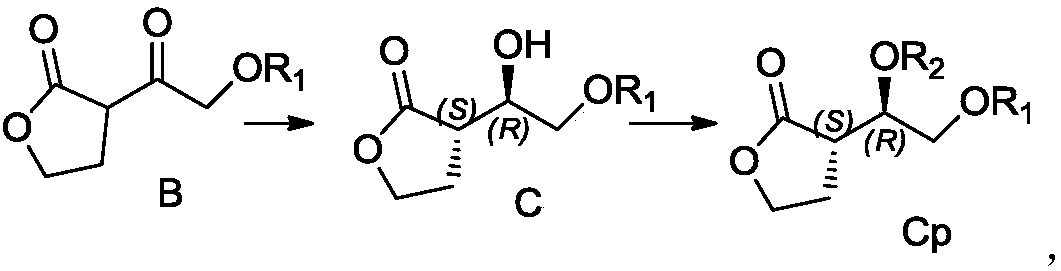
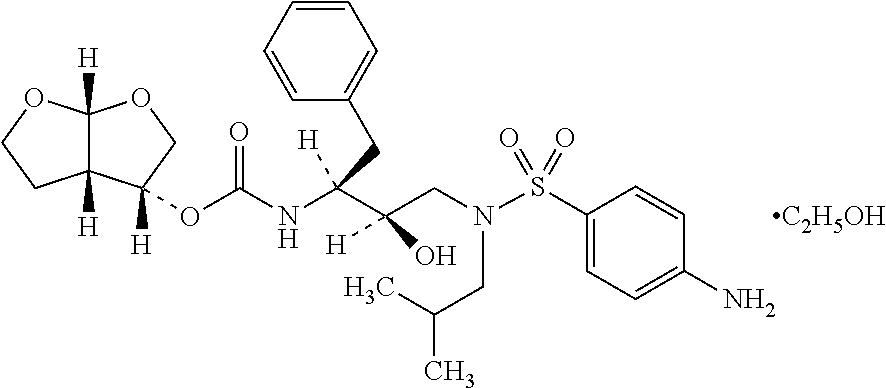
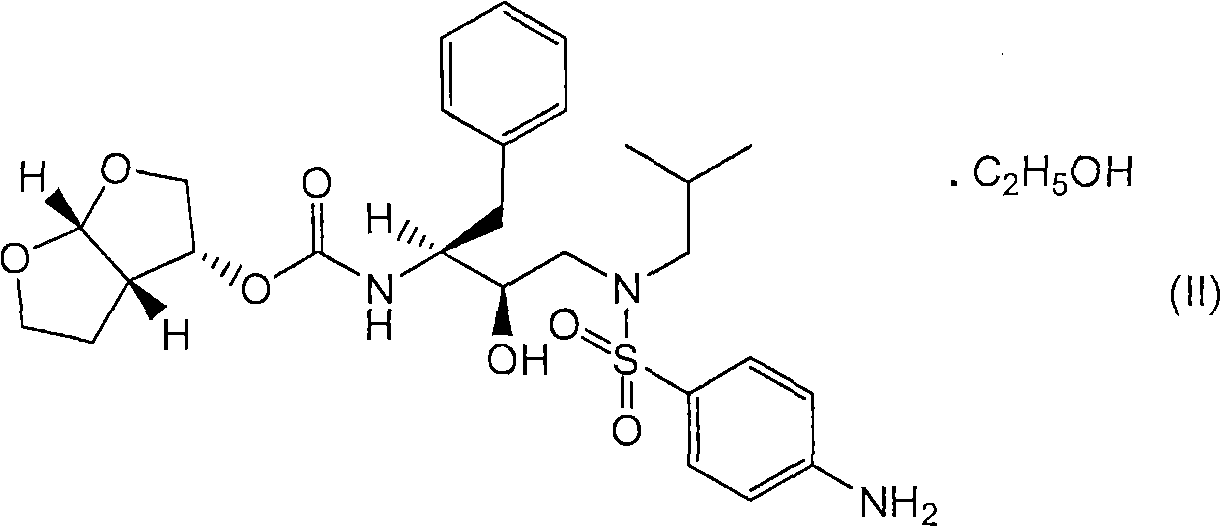
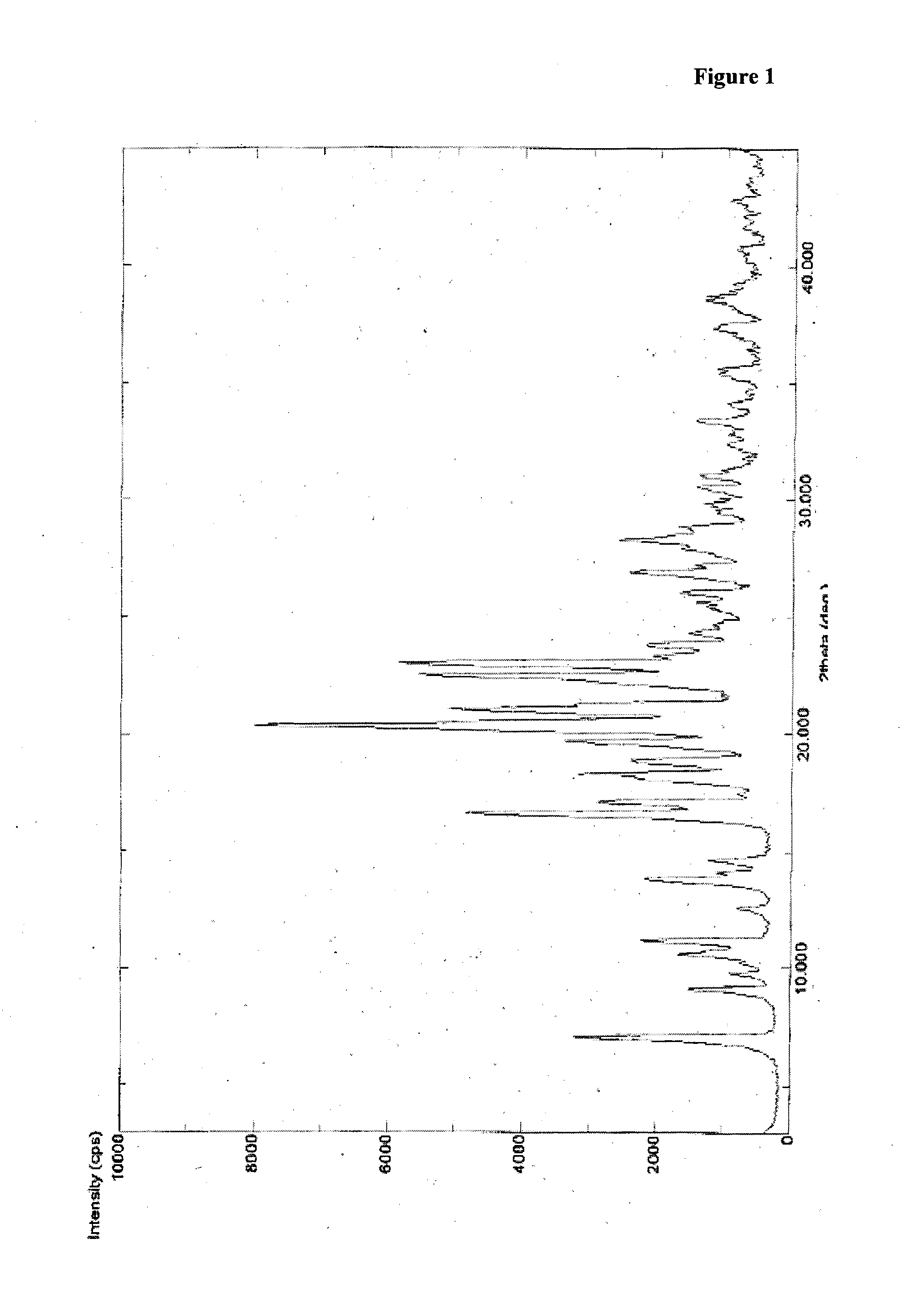
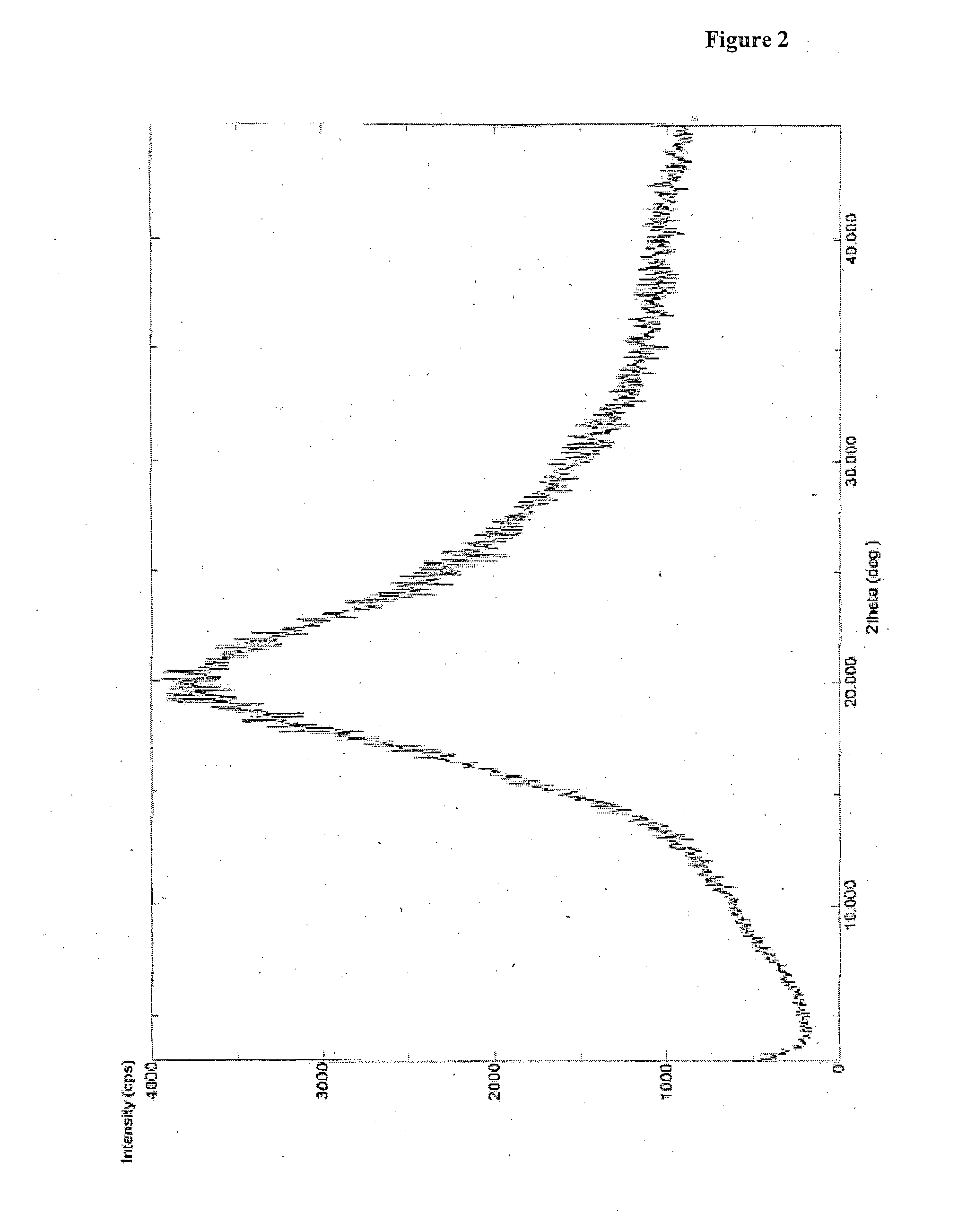
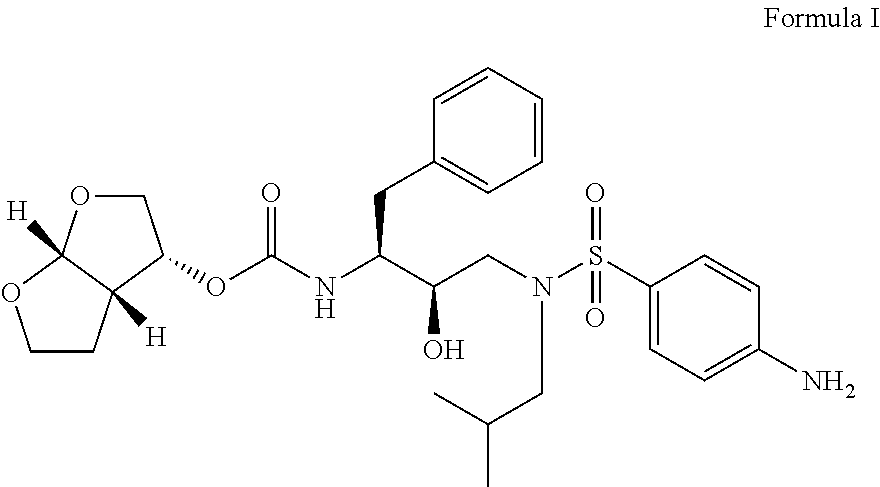
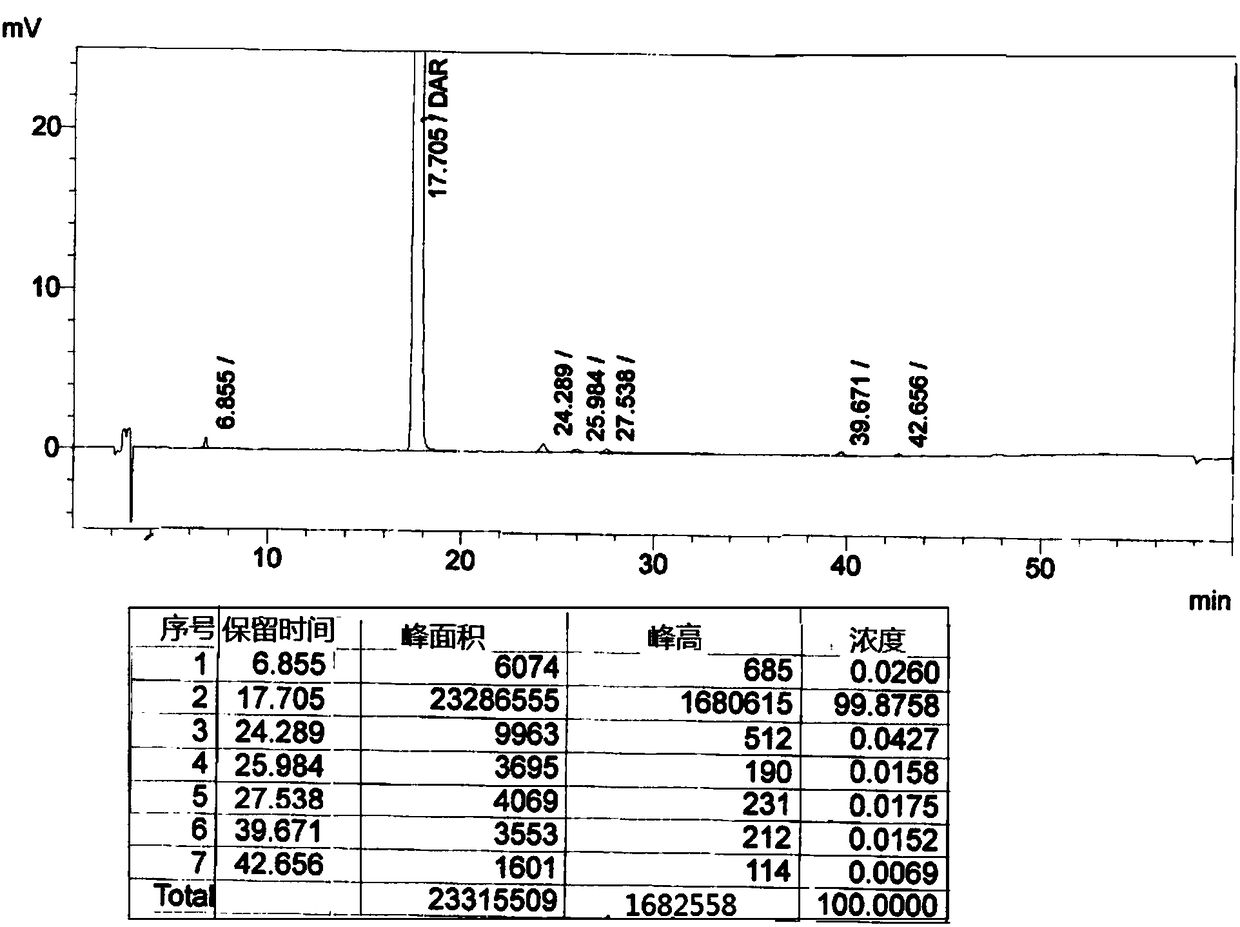
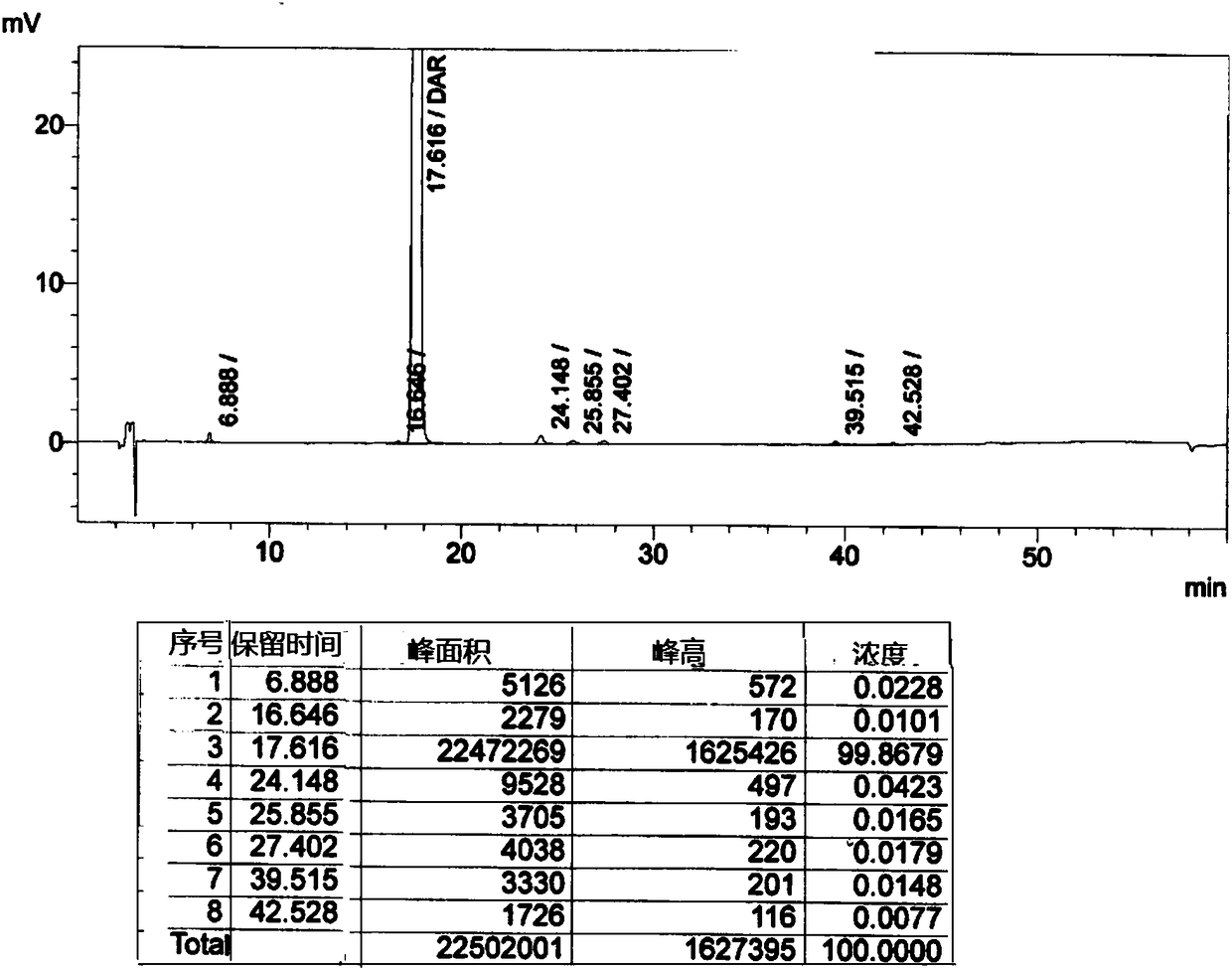
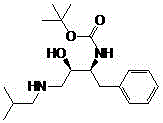
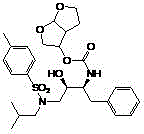
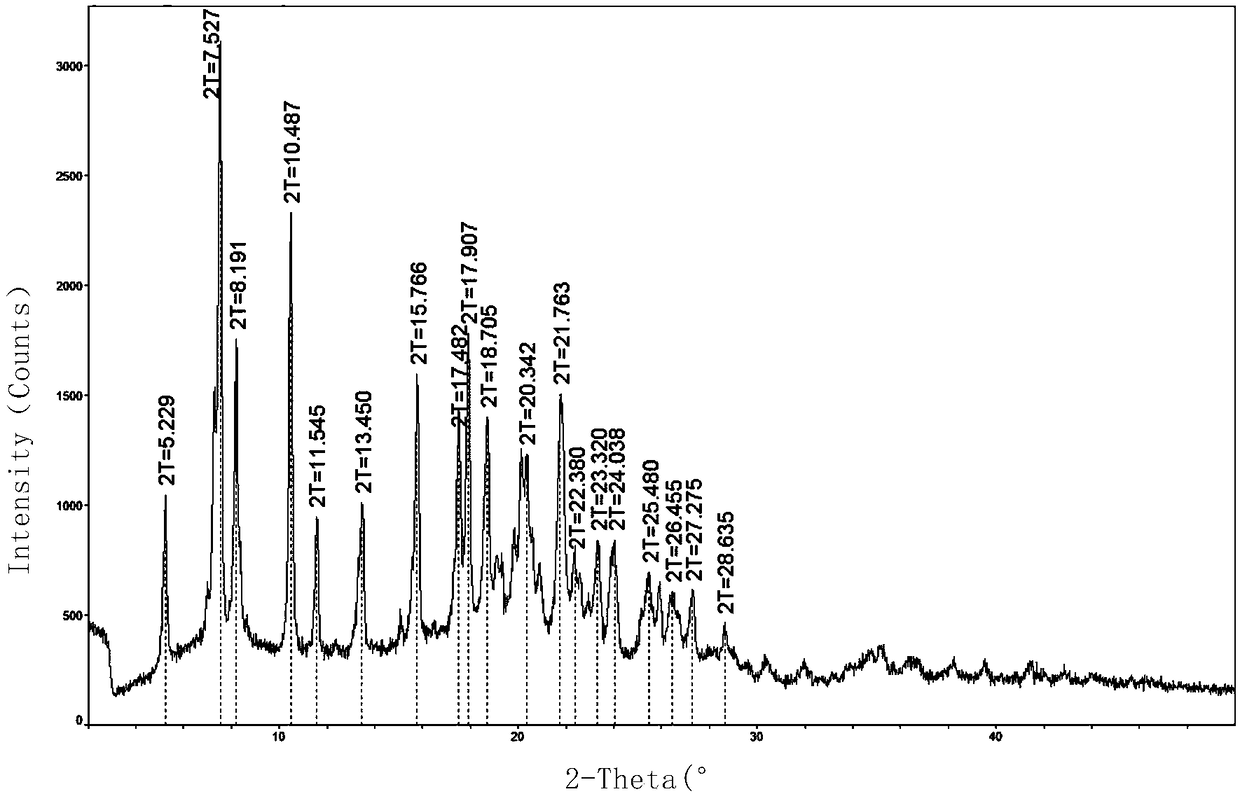
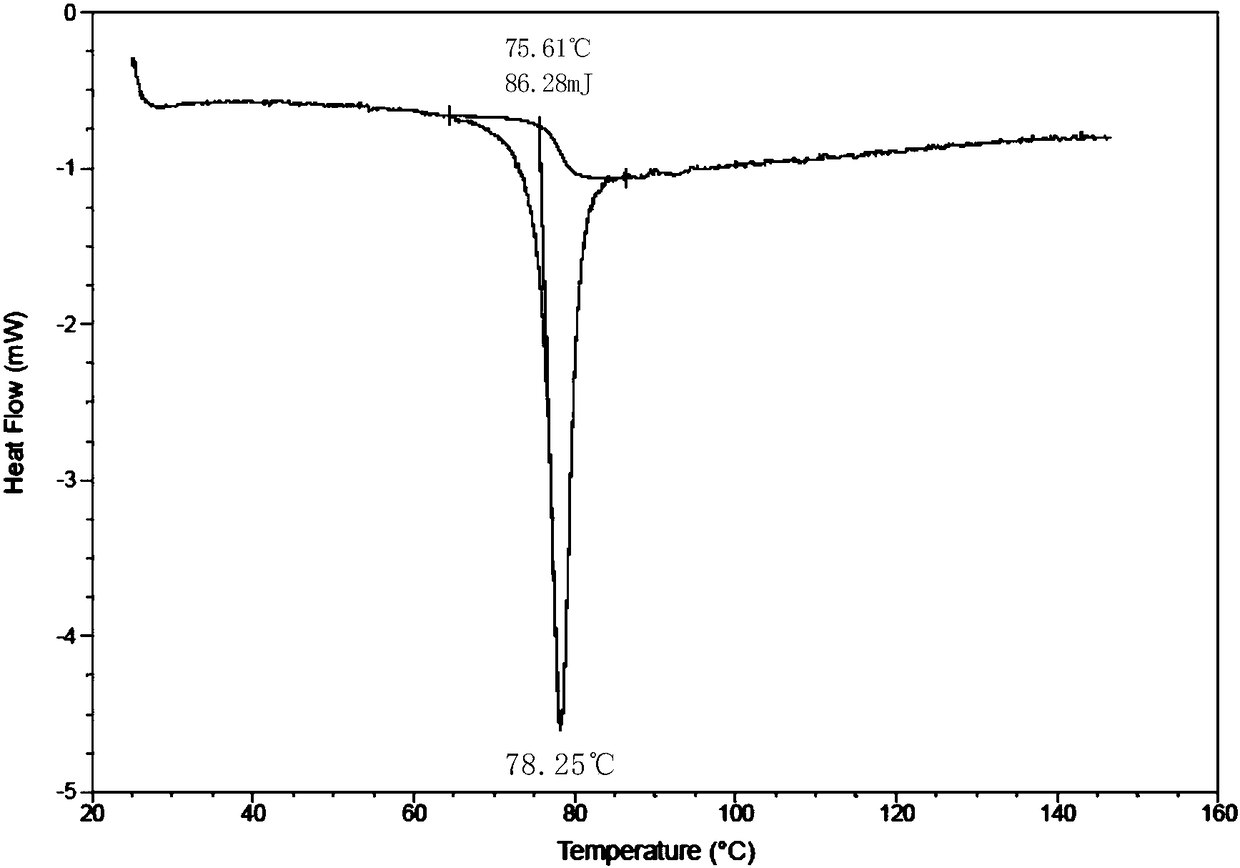
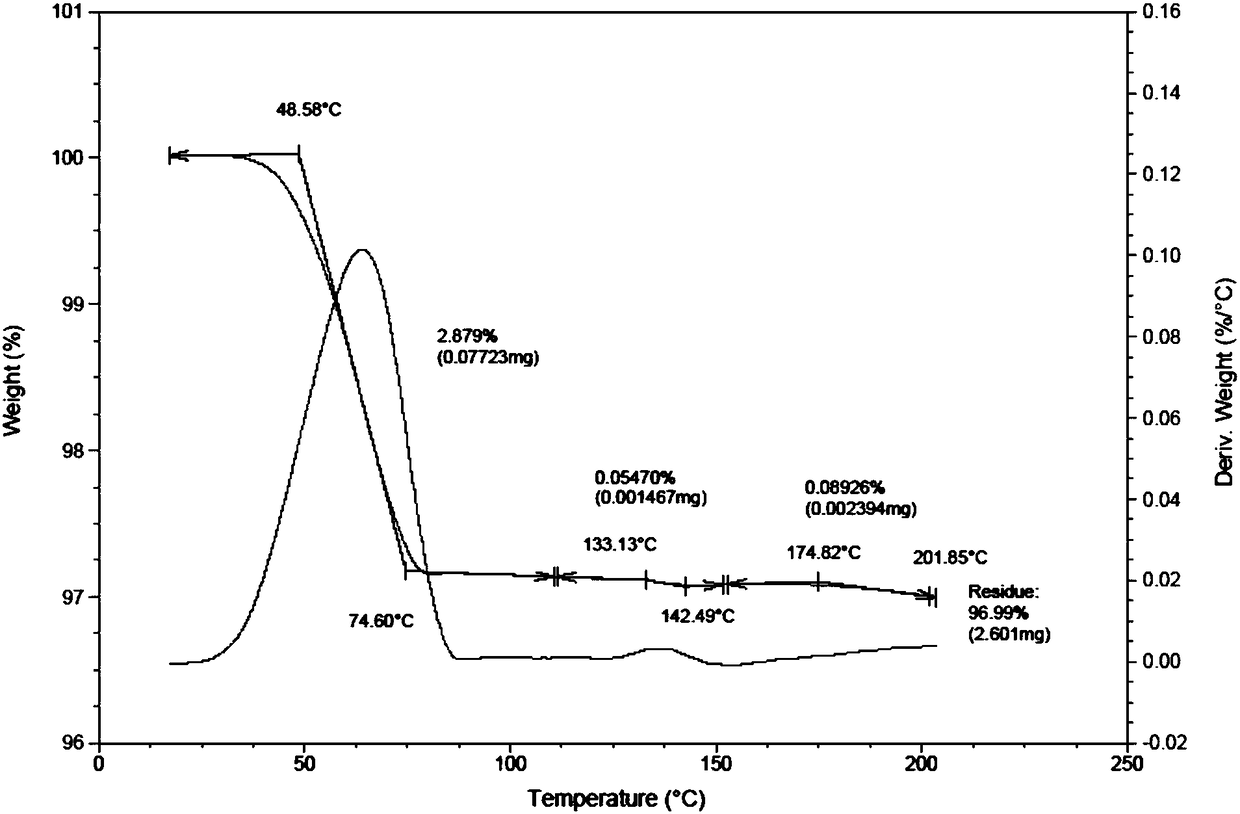
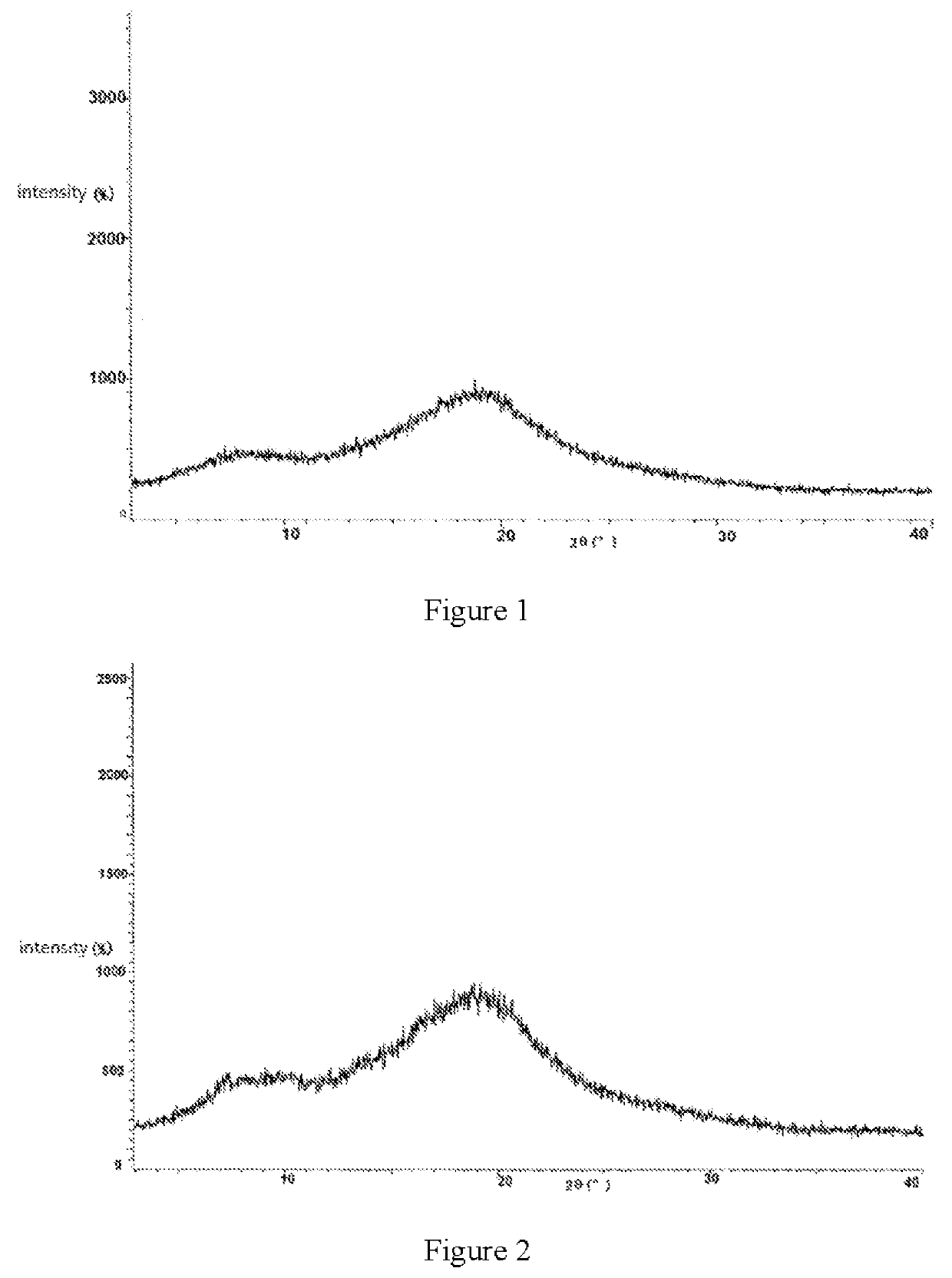
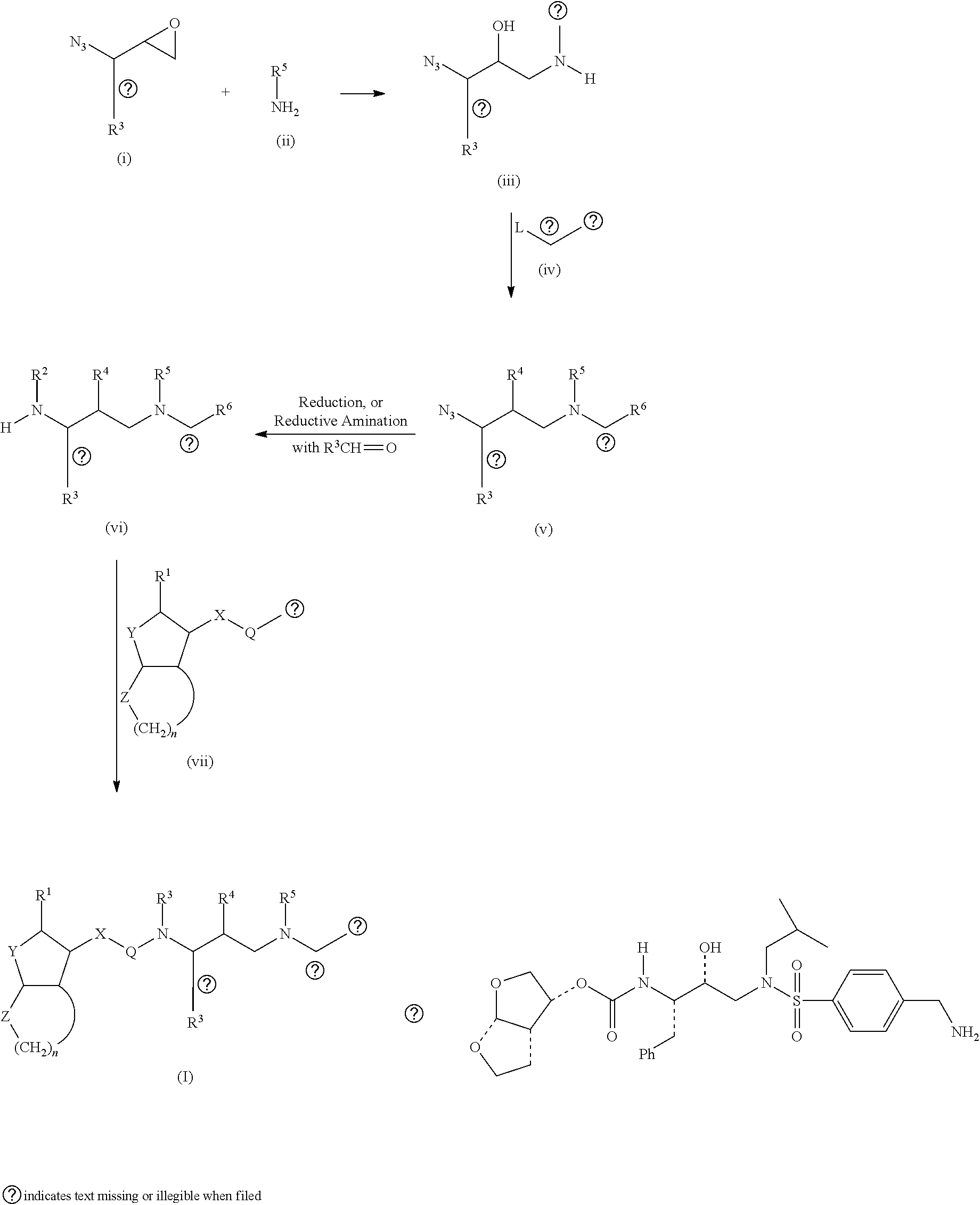Patents
Literature
38 results about "Darunavir+Ritonavir" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
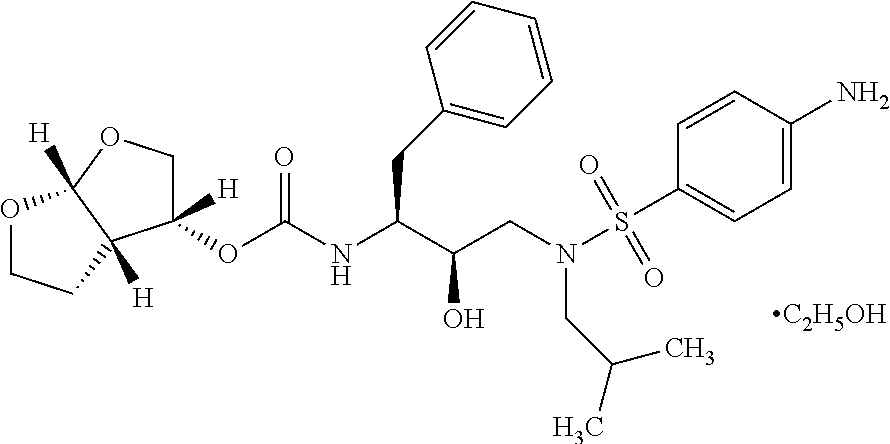
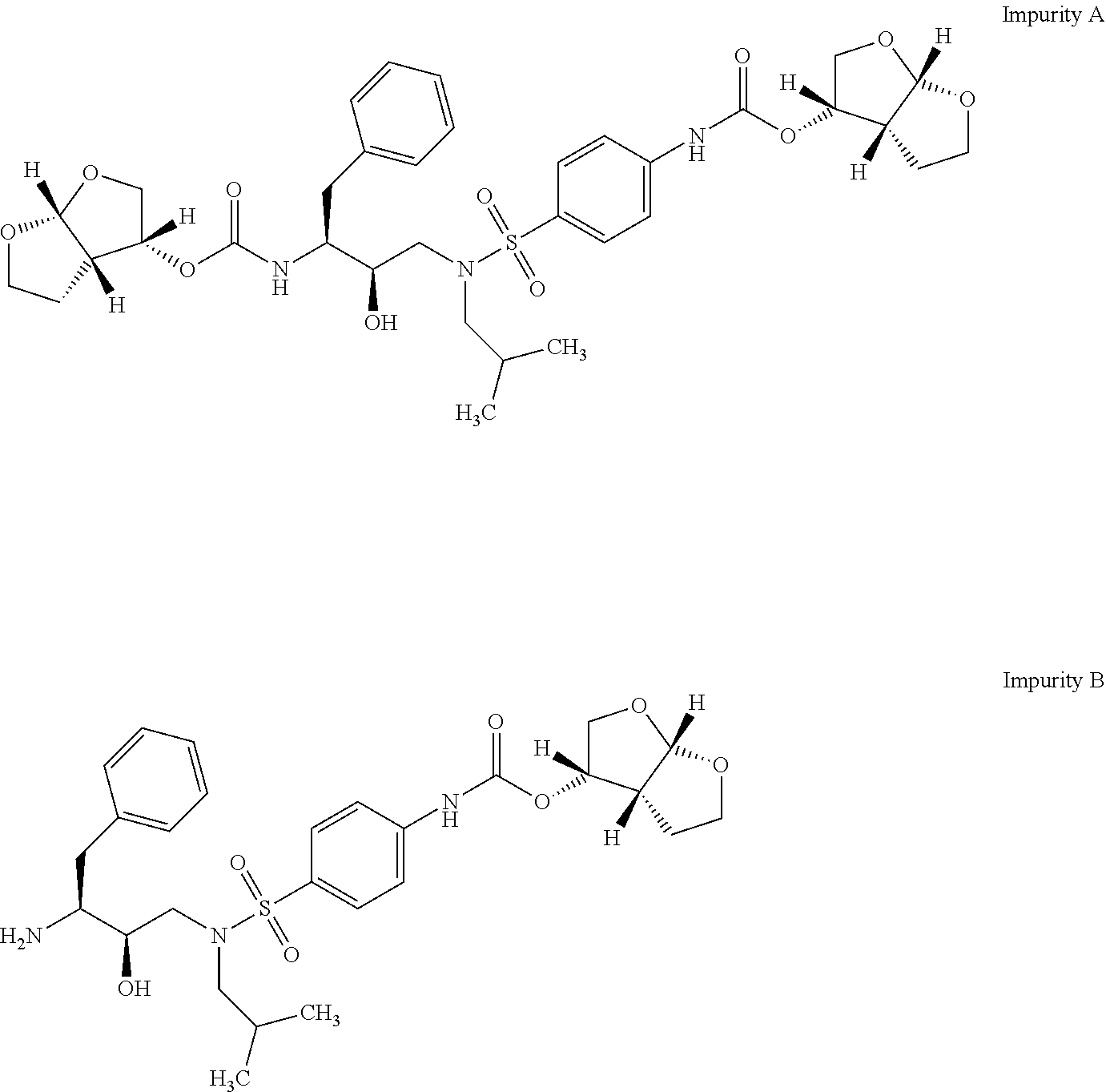
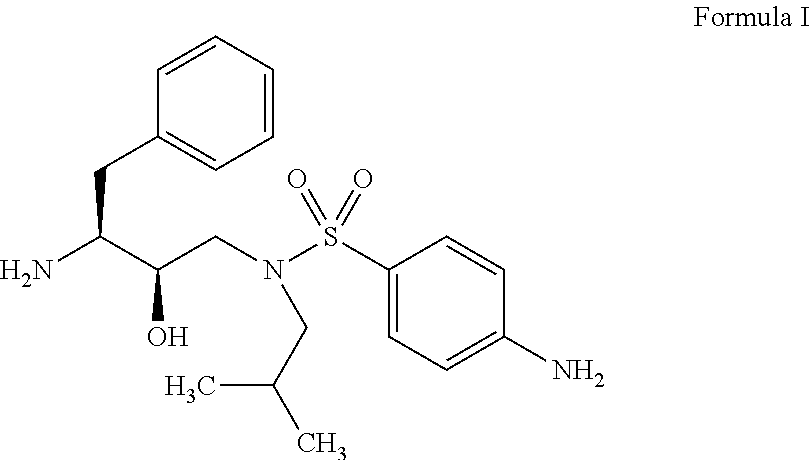
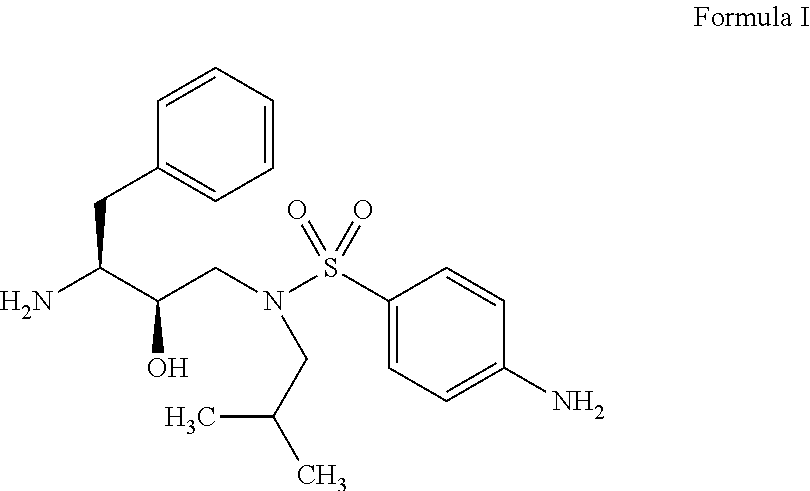
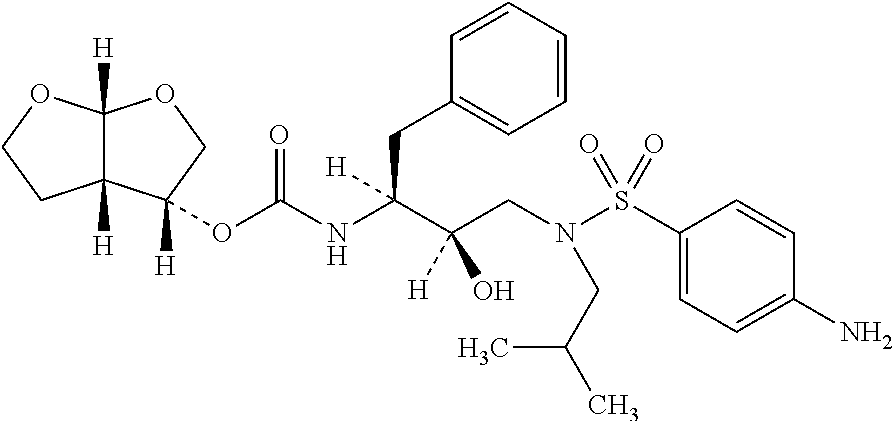
Darunavir (DRV), sold under the brand name Prezista among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It is often used with low doses of ritonavir or cobicistat to increase darunavir levels.
Darunavir combination formulations
InactiveUS20140142070A1Less frequentBiocideOrganic active ingredientsDarunavir+RitonavirSolid oral dosage form
Owner:GILEAD SCI INC +1
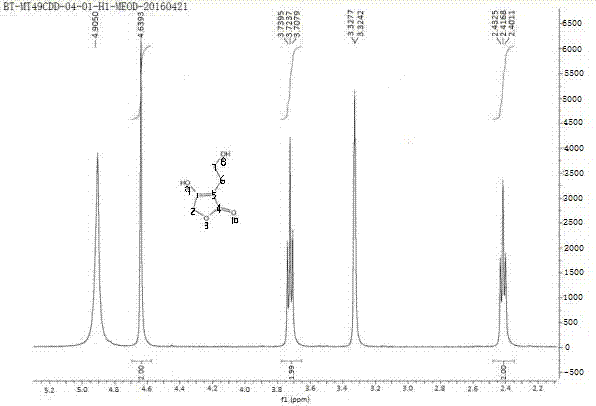
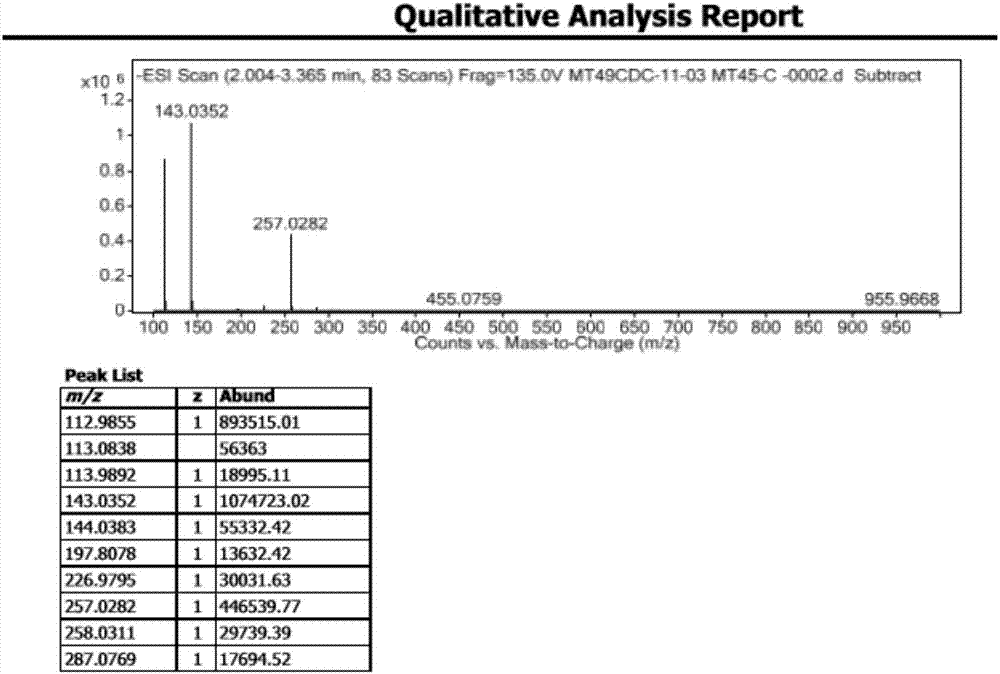
Preparation method of Darunavir intermediate
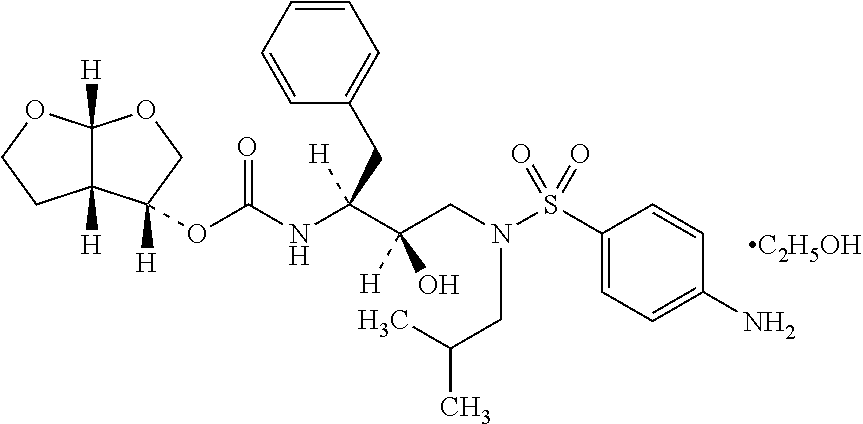
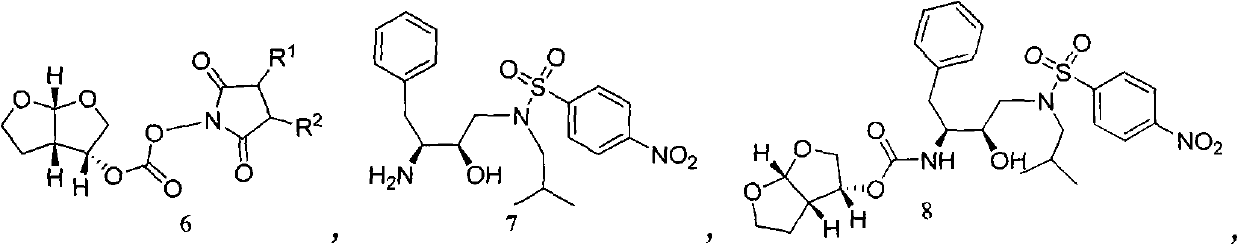
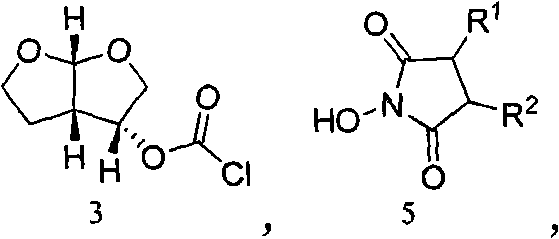
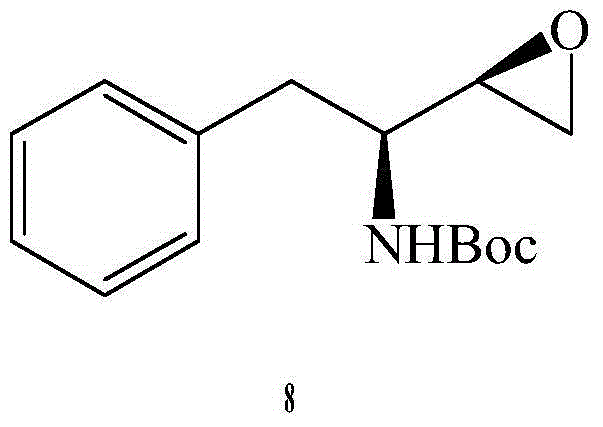
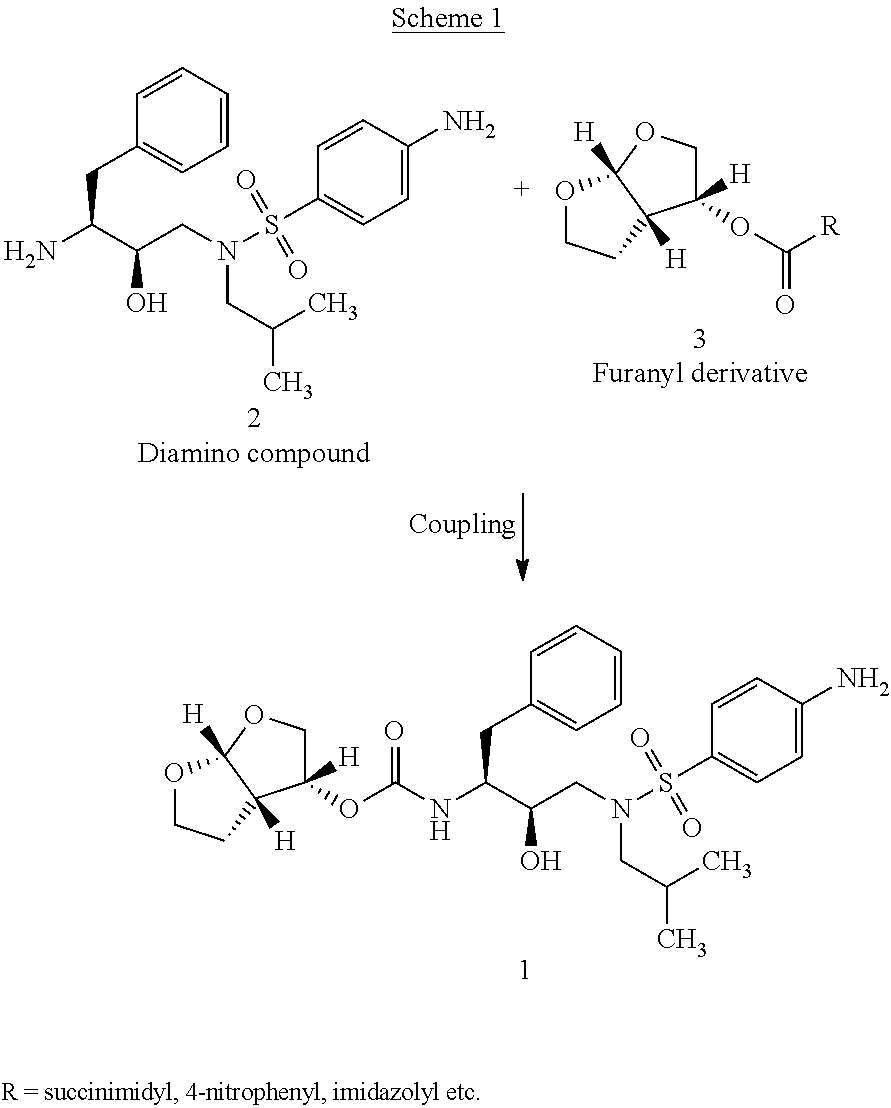
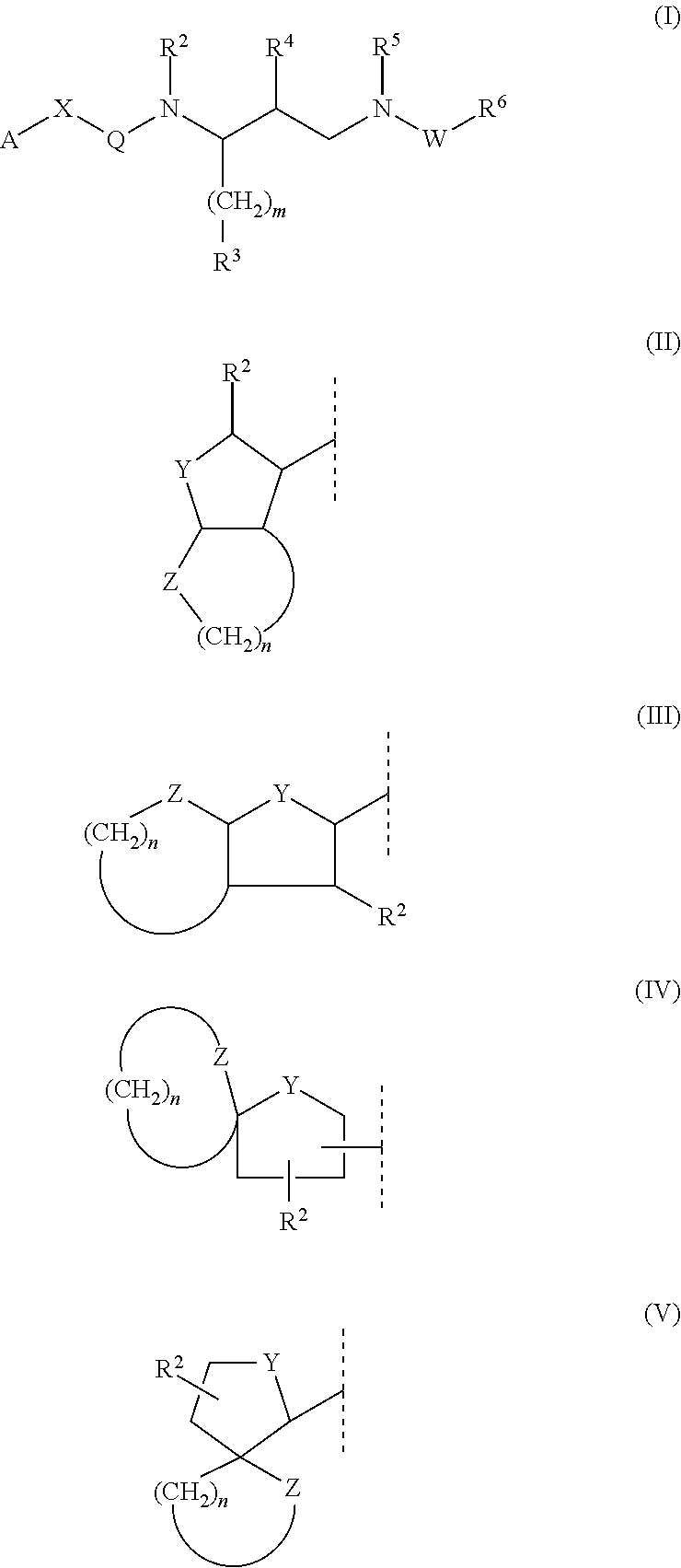
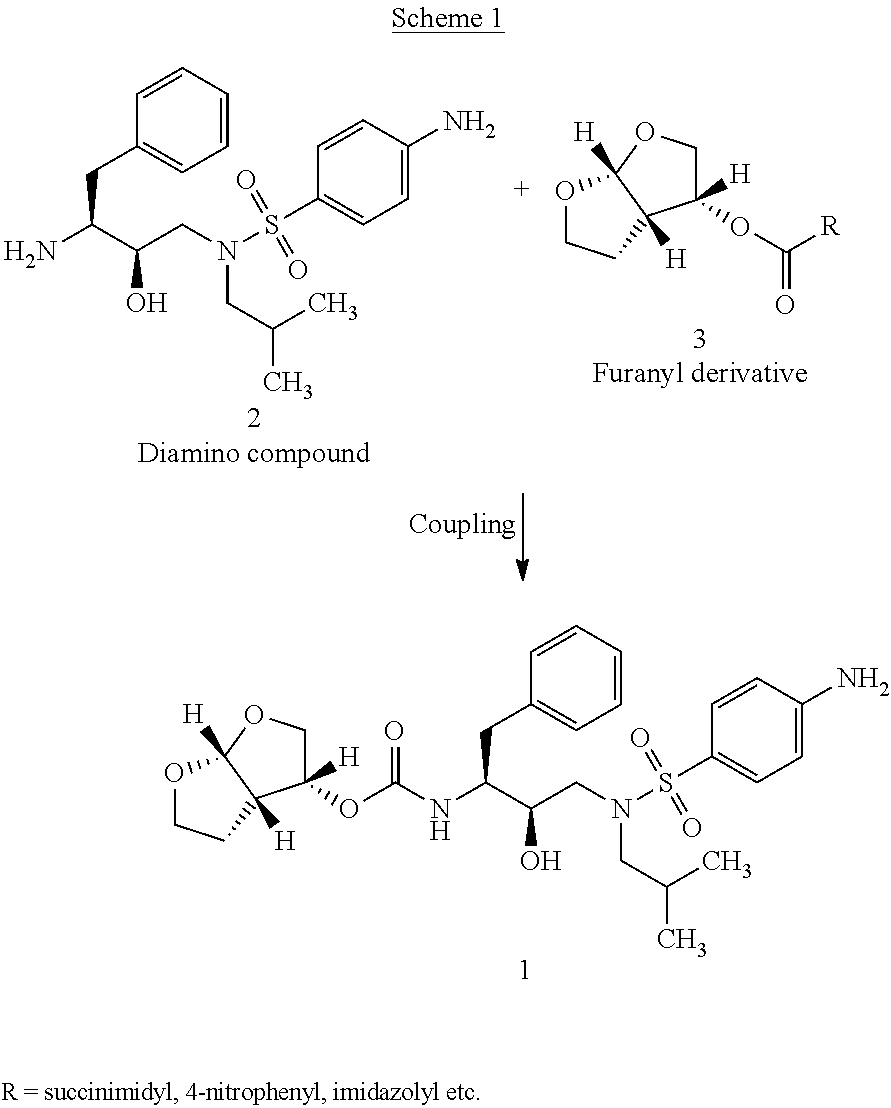
The invention relates to the technical field of heterocyclic chemistry, especially relates to a condensed ring system containing oxygen atoms as the only heterocycle atoms, concretely discloses a preparation method of a Darunavir intermediate. The method comprises the following steps: obtaining a compound of formula (3) by using (3R,3aS,6aR)-hexahydro-furo[2,3-b]furan-3-ol as a raw material to react with triphosgene under alkaline conditions, then directly reacting with a compound of formula (7) to obtain a compound of formula (8); or obtaining a compound of formula (3) to react with an N-hydroxyl compound to prepare active ester, and then reacting with the compound of formula (7) to obtain the compound of formula (8).
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
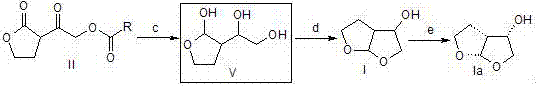
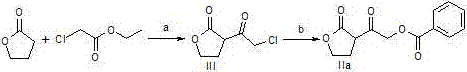
Darunavir midbody as well as preparation method and application thereof
InactiveCN103896886ALow costRaw materials are cheap and easy to getOrganic chemistryArylChemical structure
The invention discloses a darunavir midbody as well as a preparation method and application thereof. The midbody has a chemical structure shown by a formula II which is as shown in the specification. The preparation method of the midbody comprises the step b or step a-b of the synthetic route, wherein X is selected from Cl, Br or I, R is selected from aryl or alkyl, and R1 is selected from the alkyl of C1-C4. The midbody can be used for preparing the key midbody of darunavir, namely, hexahydrofuran-[2,3-b] furan-3-alcohol and (3R,3aS,6aR) hexahydrofuran-[2,3-b] furan-3-alcohol. The midbody has the advantages of using cheap and easily-available raw materials, being simple to prepare, having low cost, and being applicable to large scale production, thus having significance and practical value to realization of industrial production of darunavir.
Owner:SHANGHAI DESANO CHEM PHARMA
Unit dosage form comprising emtricitabine, tenofovir, darunavir and ritonavir and a monolithic tablet comprising darunavir and ritonavir
The present invention relates to an oral unit dosage form comprising Emtricitabine, Tenofovir, Darunavir and Ritonavir and a monolithic tablet comprising Darunavir and Ritonavir and their use to treat HIV infection.
Owner:TEVA PHARMA IND LTD
Preparation method for hexahydrofurofuranol derivative, intermediate of hexahydrofurofuranol derivative, and preparation method for intermediate
ActiveCN110272398AHigh optical purityHigh yieldOrganic chemistry methodsOxidoreductasesDarunavir+RitonavirCombinatorial chemistry
The invention relates to the field of pharmaceutical synthesis, specifically to a preparation method for a hexahydrofurofuranol derivative, an intermediate of the hexahydrofurofuranol derivative, and a preparation method for the intermediate. According to the preparation method provided by the invention, a compound with a formula A1 which is shown in the description is used as a starting raw material; chirality is established through an enzymatic method in the preparation process of the hexahydrofurofuranol derivative; thus, by adoption of the technical means of the invention, a product with high optical purity can be prepared. The preparation method provided by the invention can realize commercial production and preparation of a darunavir key intermediate namely (3R,3aS,6aR)-hexahydrofuro[2,3-b]-3-ol, and is an economical route applicable to industrial production.
Owner:JIANGSU RUIKE MEDICAL SCI & TECH CO LTD
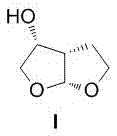
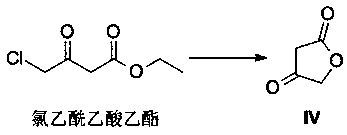
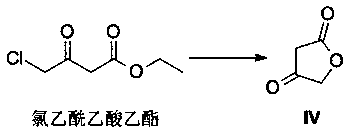
A method of preparing a darunavir intermediate
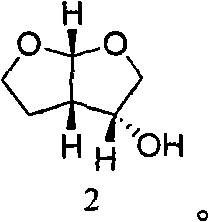
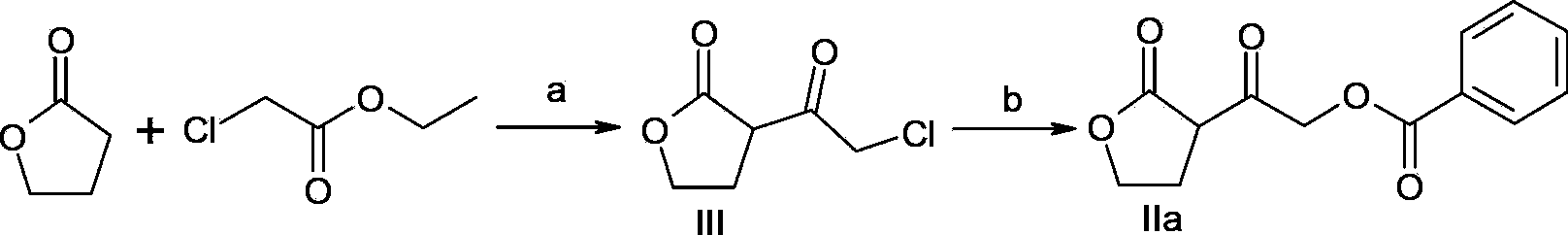
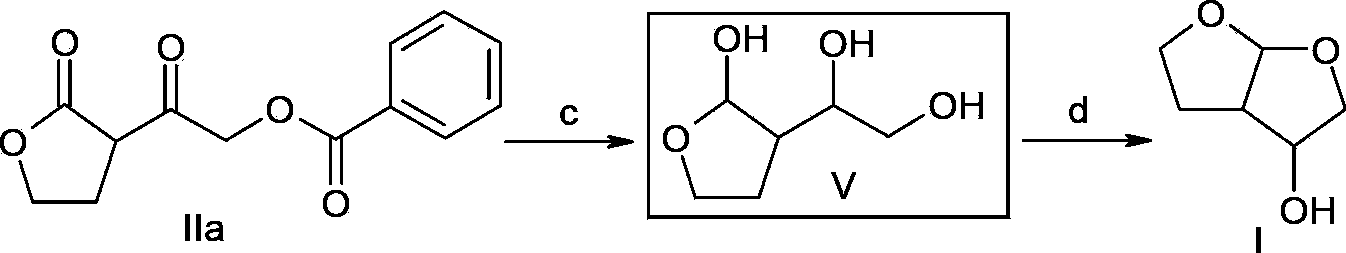
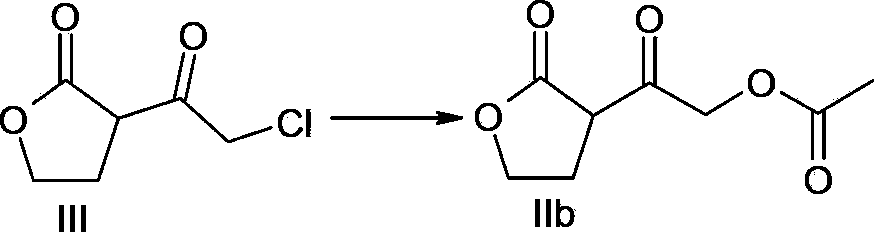
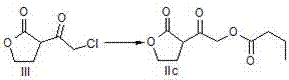
A method of preparing a darunavir intermediate is disclosed. Ethyl 4-chloroacetoacetate is adopted as a raw material and subjected four reaction steps to obtain the critical darunavir intermediate shown as a formula I. The method has characteristics of a reasonable process, simple operation, a low cost and a high yield. The method can be applied in the industry easily. The production efficiency is increased.
Owner:成都博腾药业有限公司
Combination formulations comprising darunavir and etravirine
This invention relates to solid oral dosage forms of the HIV inhibitors containing a combination of TMC114 and TMC125.
Owner:TIBOTEC PHARMA
Process for preparation of darunavir
ActiveUS20150203506A1Simple and cost-effective processHigh purityOrganic chemistry methodsAntiviralsPropionateDarunavir+Ritonavir
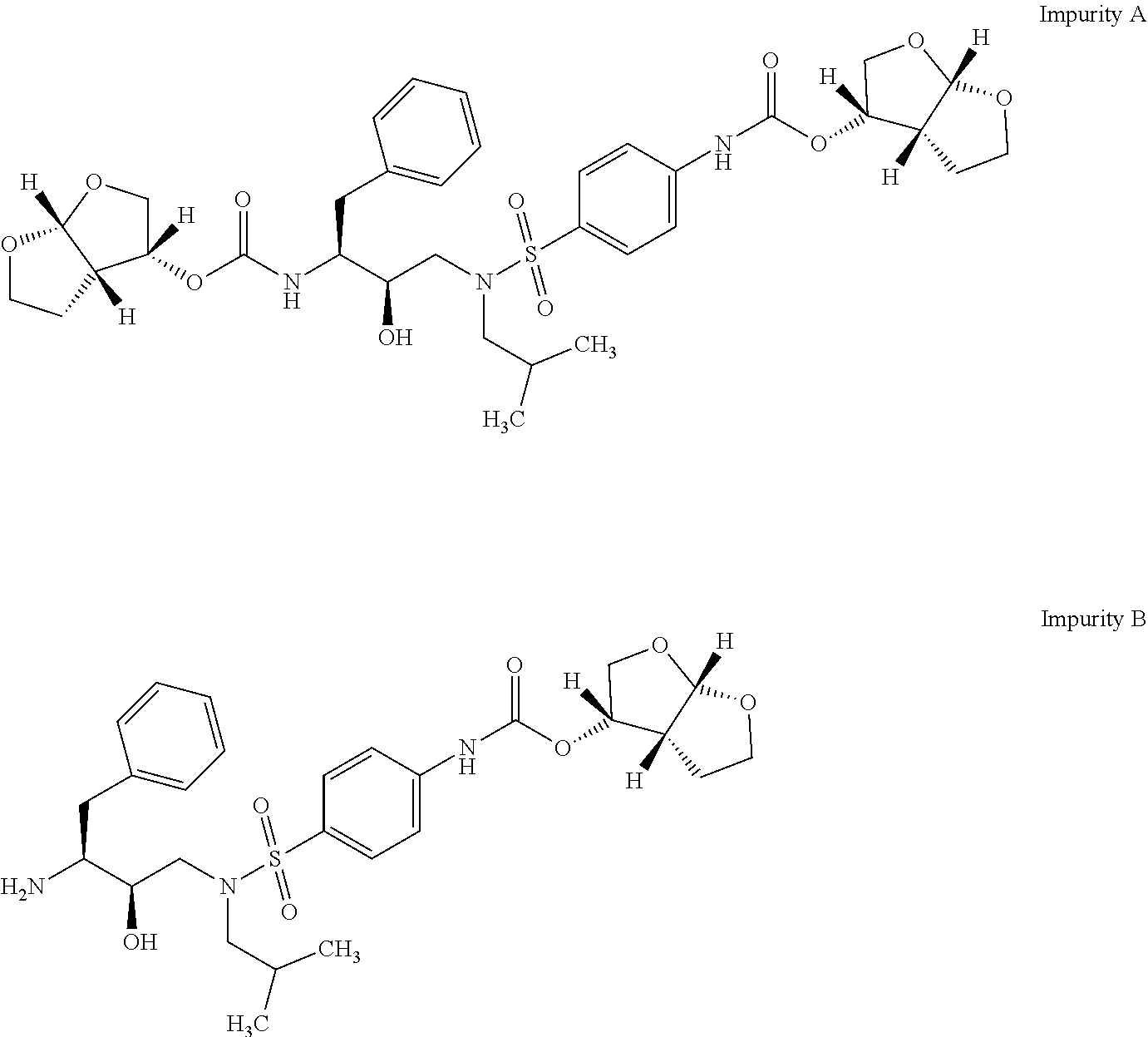
Provided are a process for preparation of darunavir or solvates or a pharmaceutically acceptable salt thereof substantially free of bisfuranyl impurities and a process for preparation of amorphous darunavir using the darunavir propionate solvate.
Owner:LAURUS LABS
Preparation method of darunavir intermediate
InactiveCN104910103ASignificant technological progressReasonable workmanshipOrganic chemistryAlkyl transferDarunavir+Ritonavir
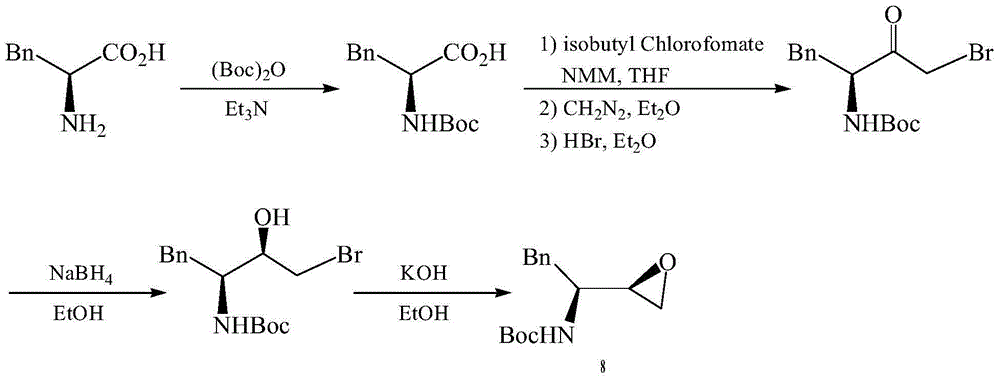
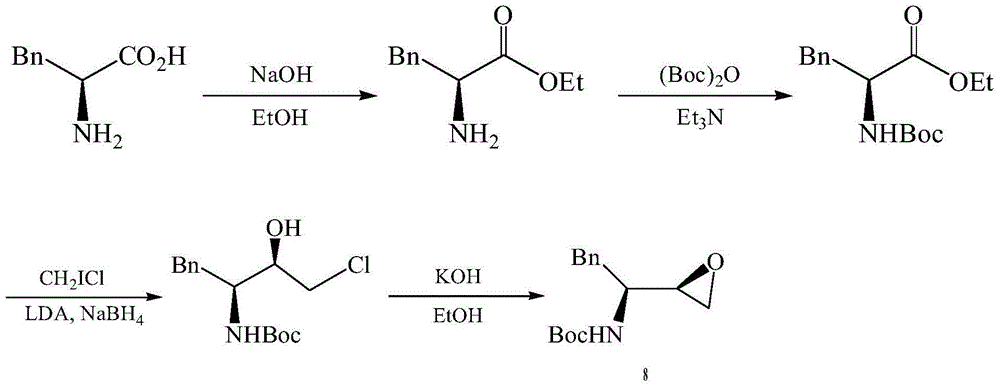
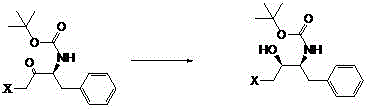
The invention provides a preparation method of a darunavir intermediate. The method adopting L-phenylalanine as a raw material comprises the steps of esterification, alkylation, acylation, palladium-carbon reduction and cyclization to synthesize (2R,3S)-1,2-epoxy-3-tertbutyloxycarbonylamino-4-phenylbutane. The method adopting a cheap benzyl group to protect an amino group has the advantages of reasonable technology, simple operation, low cost, high yield, industrialization realization, and production efficiency improvement.
Owner:SHANGHAI INST OF TECH
Method for preparing Darunavir intermediate
The invention discloses a method for preparing a Darunavir intermediate and particularly relates to a preparation method of a compound of formula I shown in the description.
Owner:成都博腾药业有限公司
Medical intermediate and preparation method thereof
InactiveCN106957288ALow costRaw materials are cheap and easy to getOrganic chemistryChemical structureDarunavir+Ritonavir
The invention discloses a medical intermediate. The medical intermediate has the chemical structure shown as a formula II shown in the description, wherein the R is selected from methyl, ethyl, n-propyl, isopropyl, normal-butyl, isobutyl, tertiary butyl, sec-butyl, phenyl and naphthyl. The invention also discloses a preparation method of the intermediate; the operation is simple; the cost is low; the preparation method is suitable for scale production. The intermediate can be used for preparing a key intermediate of darunavir; important significance and practical values are realized on the industrial production of the darunavir.
Owner:YANCHENG DESANO PHARMA CO LTD
Methods and Compositions for Determining Altered Susceptibility of HIV-1 to Protease Inhibitor Treatment
InactiveUS20100227313A1Increased susceptibilityMicrobiological testing/measurementDisease diagnosisDarunavir+RitonavirProteinase activity
This invention relates to methods for determining altered susceptibility of HIV-I viruses to protease inhibitors (PIs) based on the viral genotypes. The methods generally comprise detecting, in a gene encoding protease and / or gag of the HIV-I, the presence of mutations correlated with altered susceptibility to amprenavir and / or darunavir.
Owner:LAB OF AMERICA HLDG
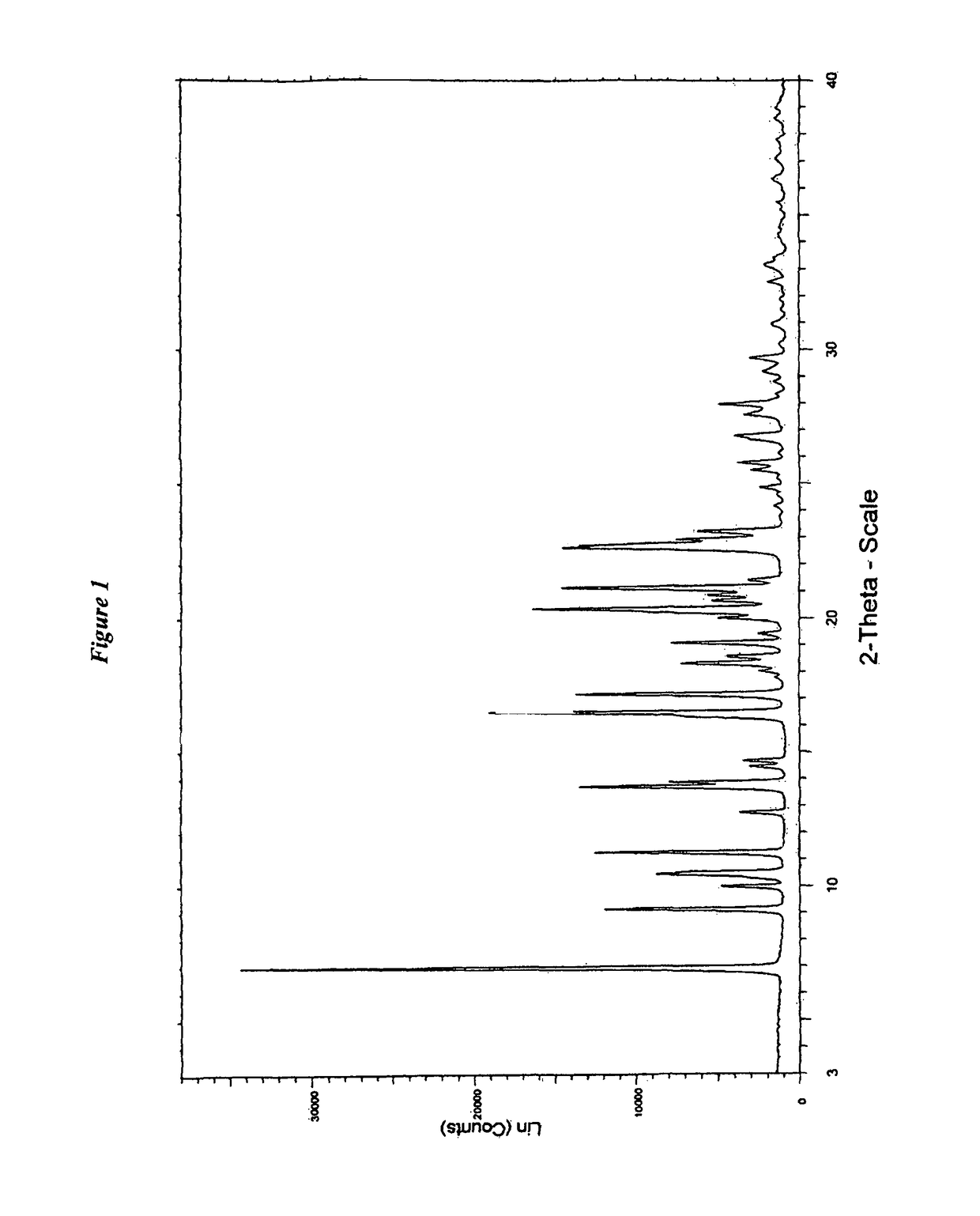
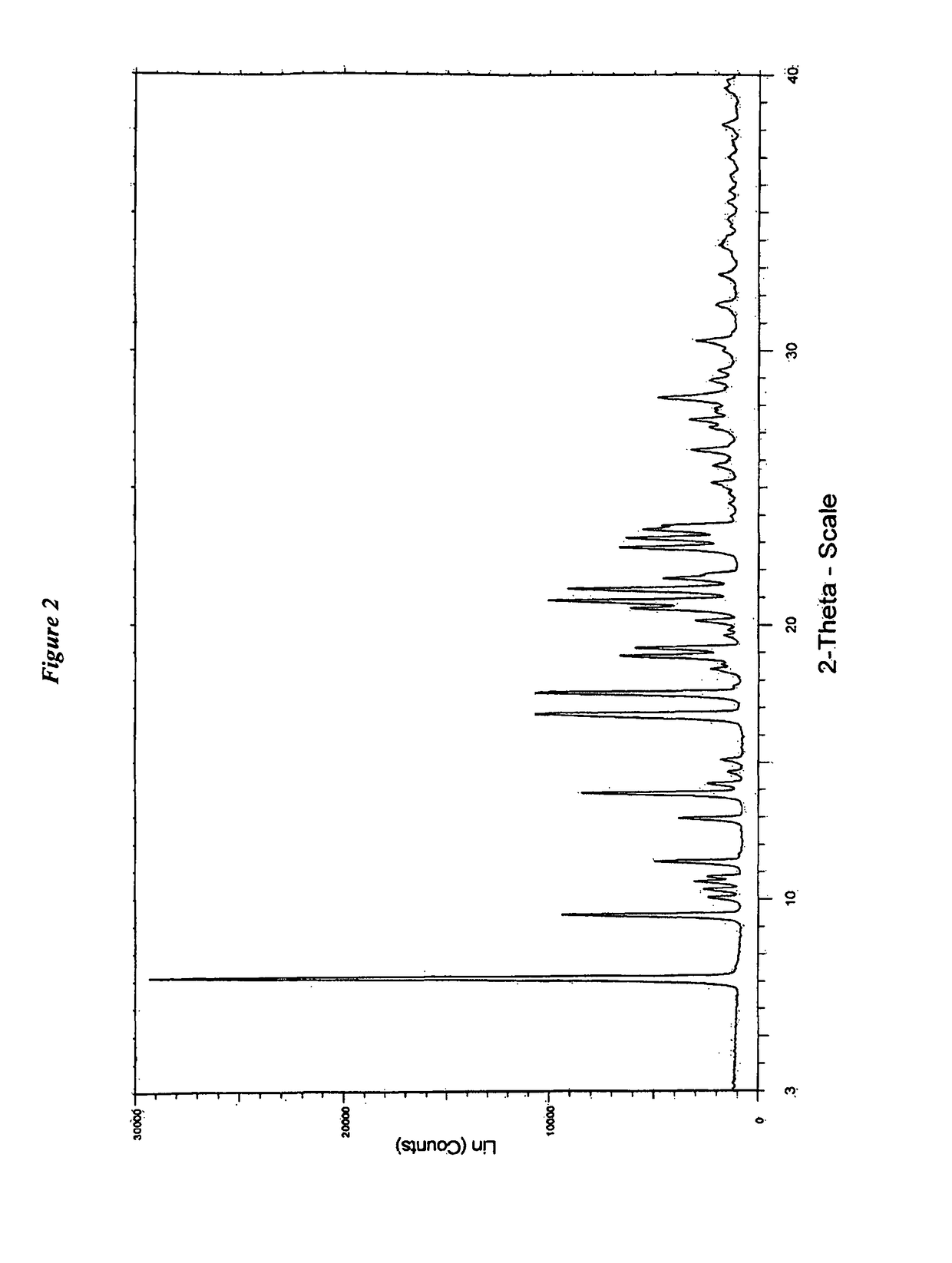
Novel process for preparation of darunavir and darunavir ethanolate of fine particle size
ActiveUS20120237770A1Organic compound preparationSynthetic resin layered productsFuranDarunavir+Ritonavir
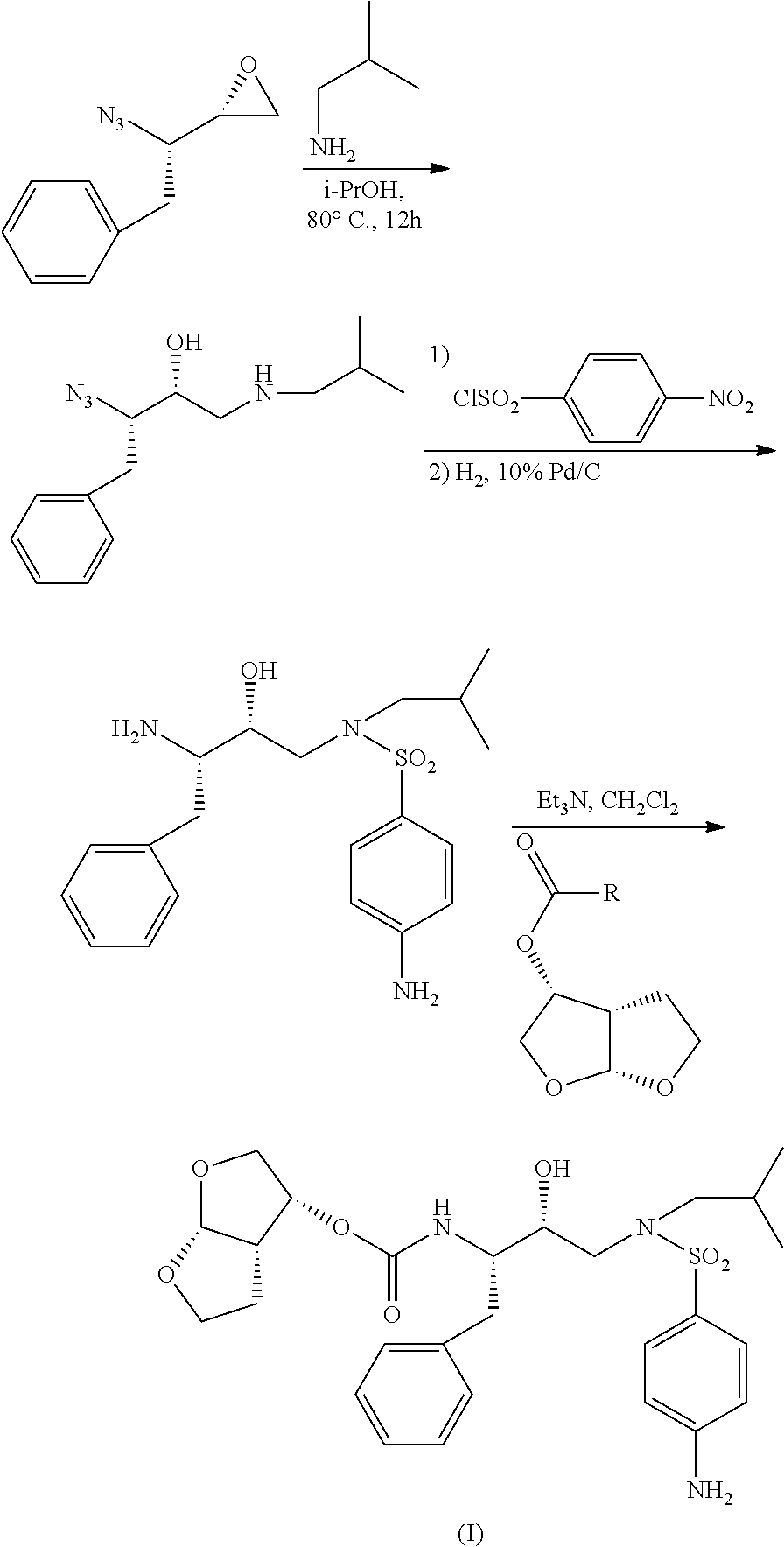
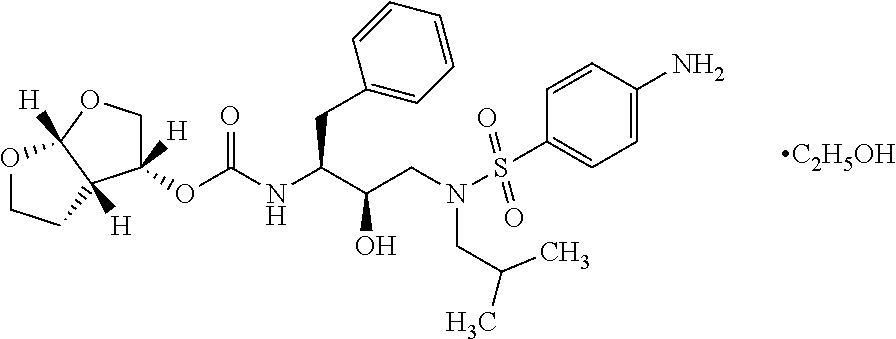
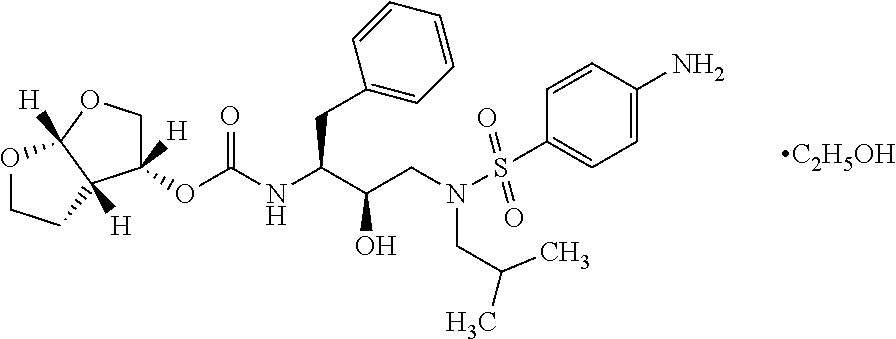
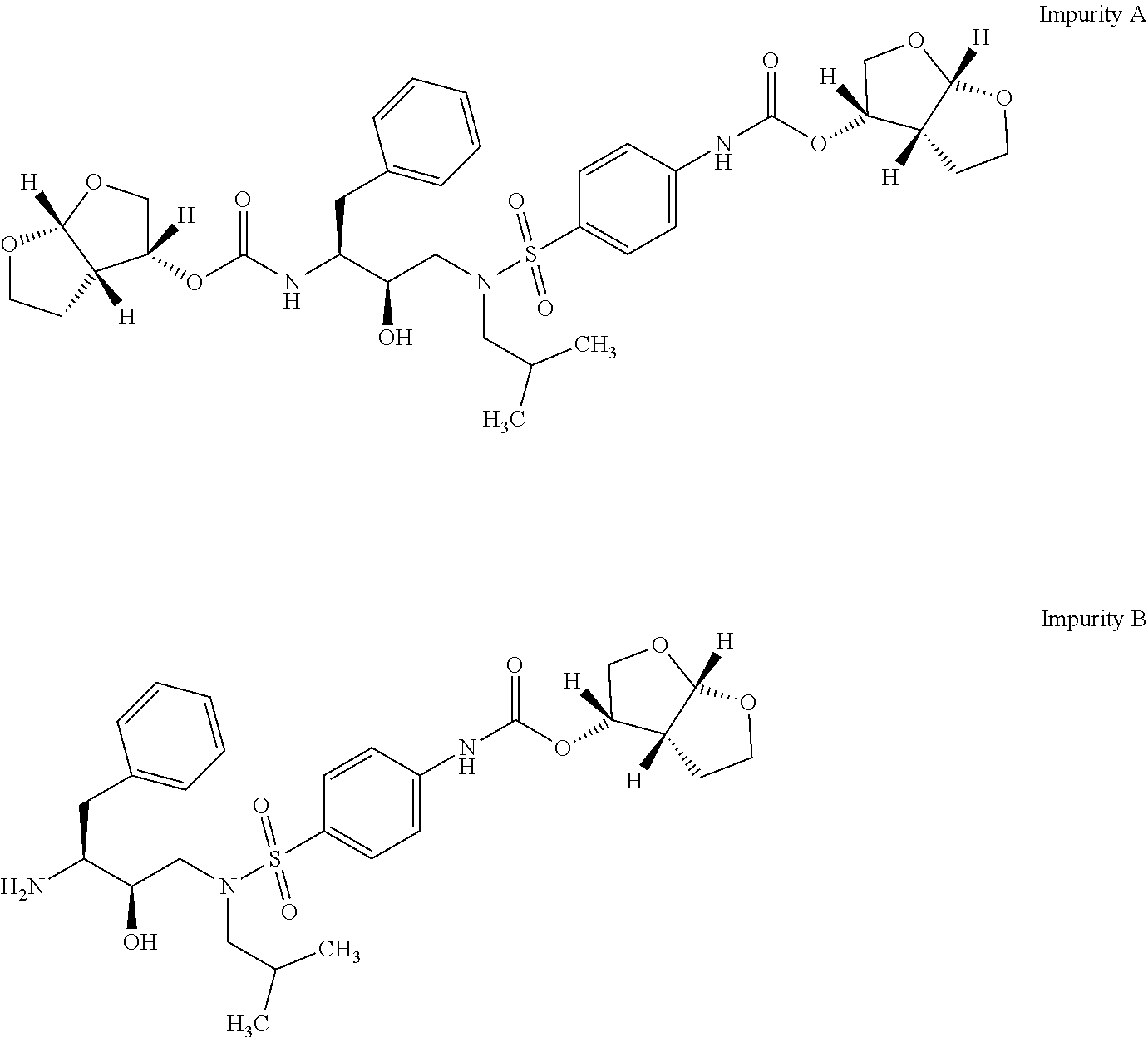
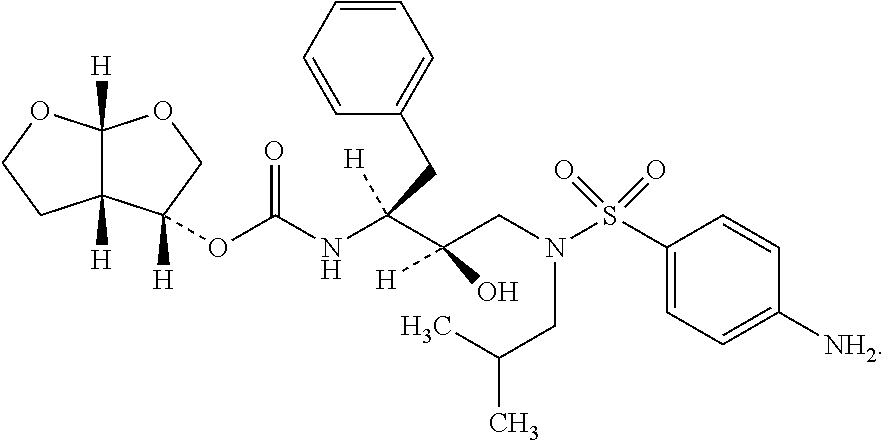
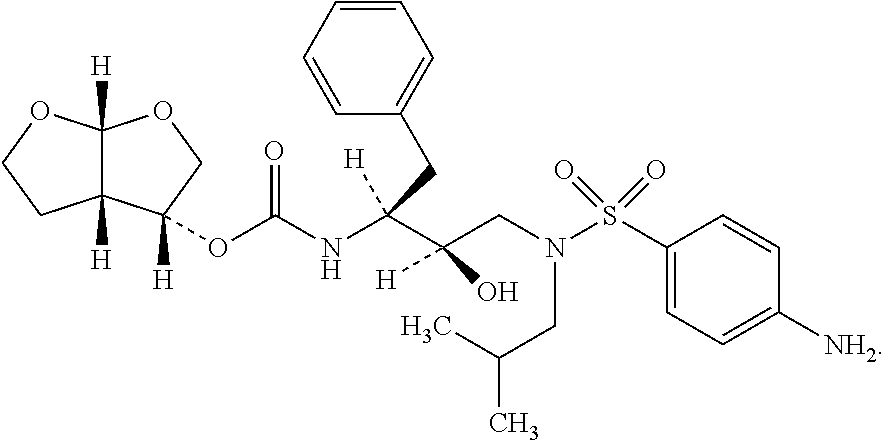
The present invention provides a novel process for preparation of darunavir that involves reduction of [(1S,2R)-3-[[(4-nitrophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy -1-(phenylmethyl)propyl]carbamic acid (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester, of formula (5). The present invention also provides darunavir ethanolate of particle size wherein d0.9 is less than 130 μm, d0.5 is less than 30 μm, d0.1 is less than 10 μm and process for its preparation.
Owner:LUPIN LTD
Process for the preparation of darunavir and darunavir intermediates
The present invention relates to a process for the preparation of darunavir, a nonpeptide protease inhibitor (PI), useful for the treatment of HIV / AIDS patients harboring multidrug-resistant HIV-1 variants that do not respond to previously existing HAART regimens. The present invention further relates to processes for the stereo-directed preparation of darunavir intermediates, in particular (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol and to certain novel intermediates obtained by such processes.
Owner:MAPI PHARMA
Quadruple therapy useful for treating persons afflicted with the human immunodeficiency virus (HIV)
InactiveUS20150305983A1Reduce in quantityMaintain curative effectComputer controlDrug and medicationsEmtricitabineNucleotide
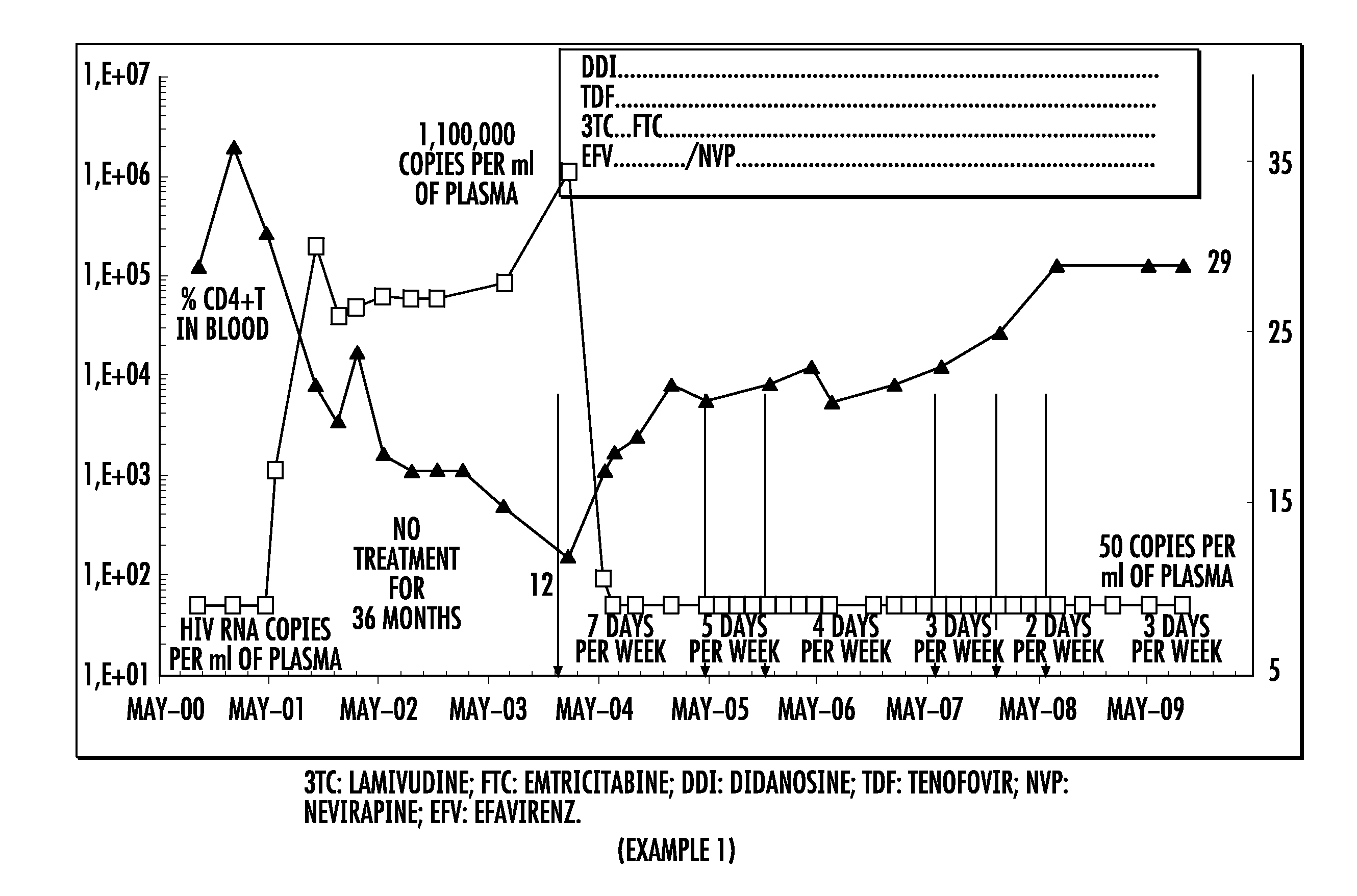
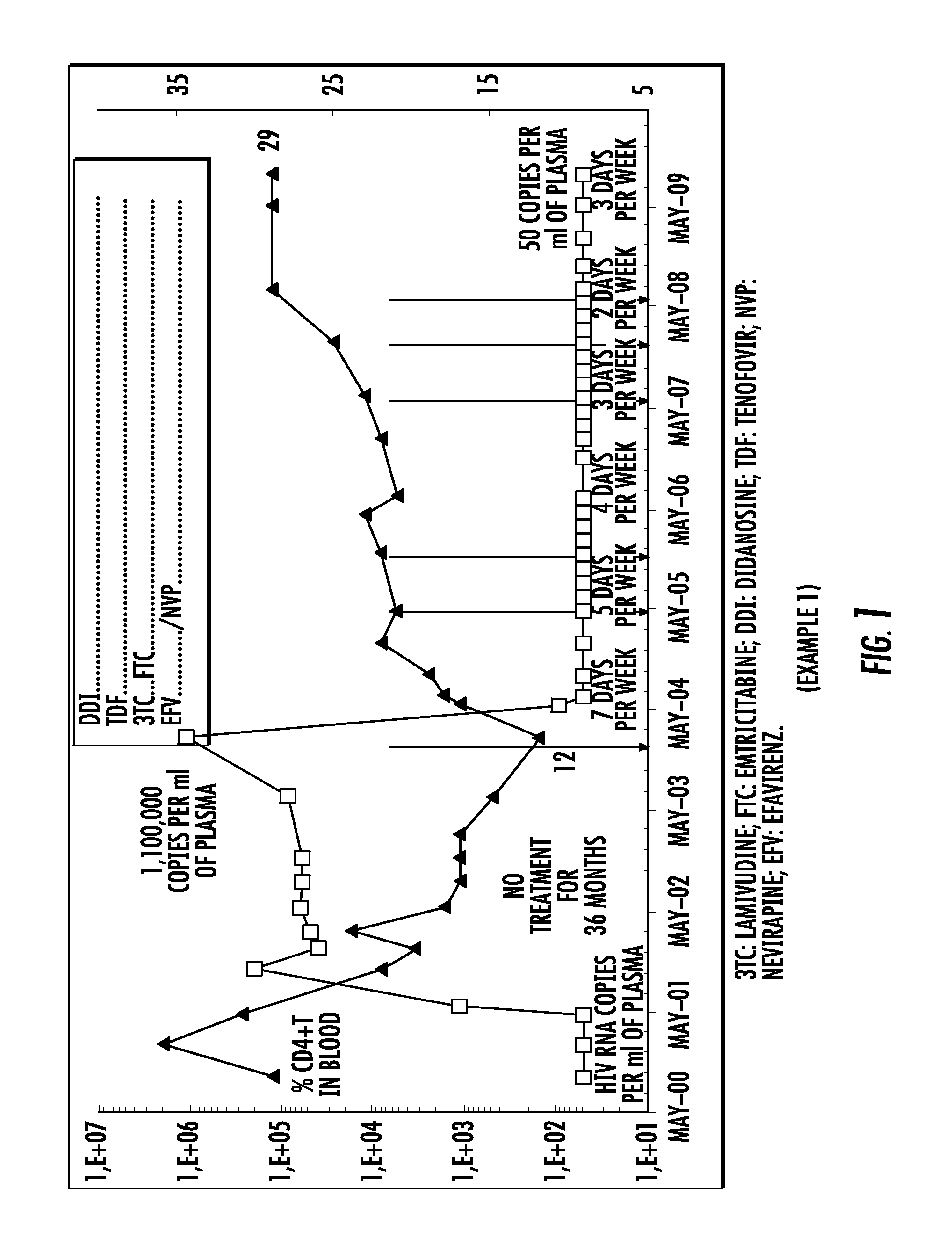
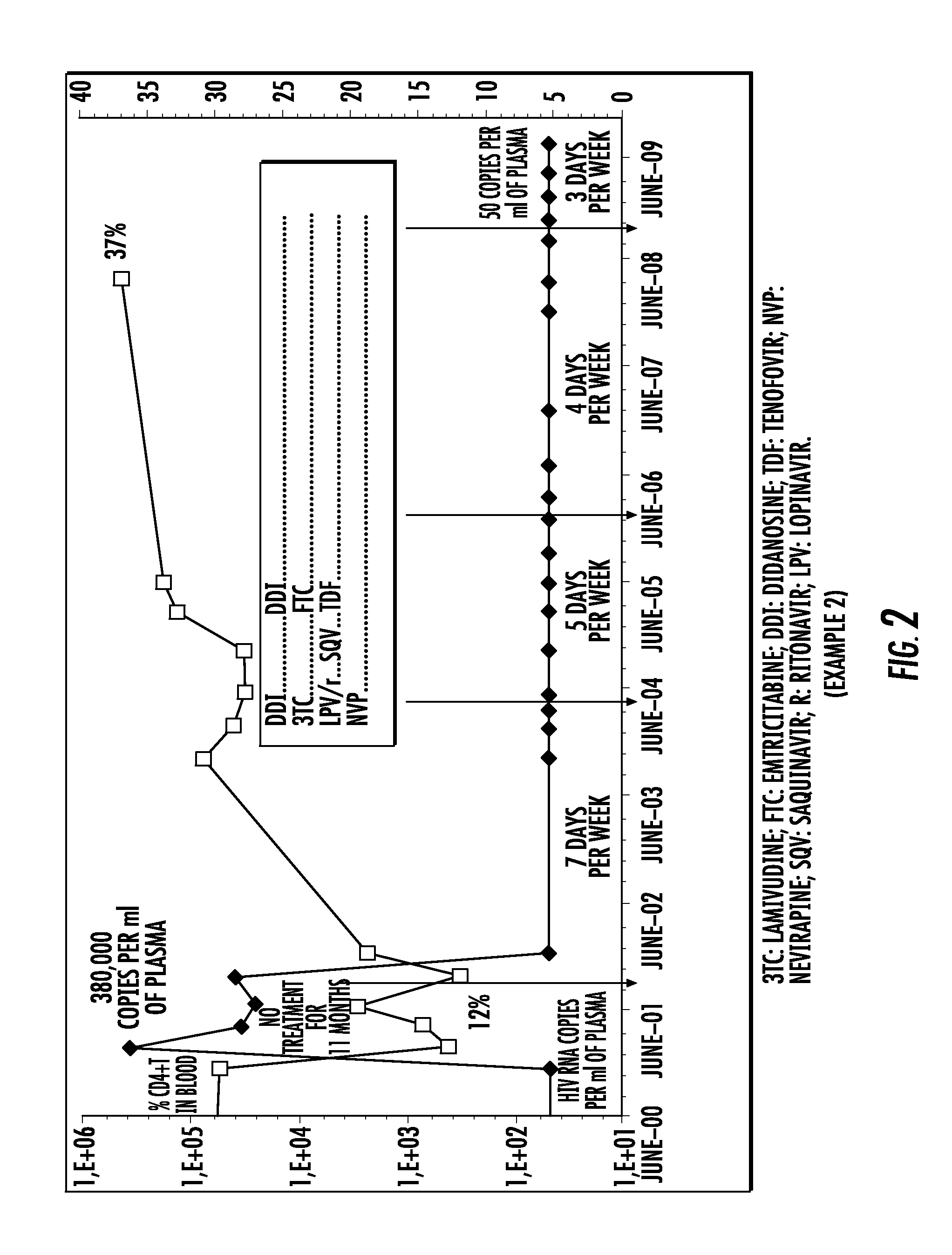
The present invention relates to a pharmaceutical composition for treating the human immunodeficiency virus (HIV) in a human being, comprising four active principles selected as being: one nucleoside inhibitor of reverse transcriptase (NRTI) selected from lamivudine and emtricitabine; two nucleoside or nucleotide inhibitor of reverse transcriptase (NRTI) selected from didanosine, abacavir and tenofovir; and the fourth active principle is selected from (i) a non-nucleoside inhibitor of reverse transcriptase (NNRTI) selected from nevirapine, efavirenz and etravirine; or (ii) a protease inhibitor selected from atazanavir, lopinavir, saquinavir, ritonavir, indinavir, amprenavir, nelfinavir, fosamprenavir, tipranavir and darunavir.The present invention also relates to an electronic portable pillbox comprising a multidrug therapy for treating the immunodeficiency virus (HIV) in human beings allowing improving the observance of medication intake.
Owner:UNIV VERSAILLES SAINT QUENTIN EN YVELINES
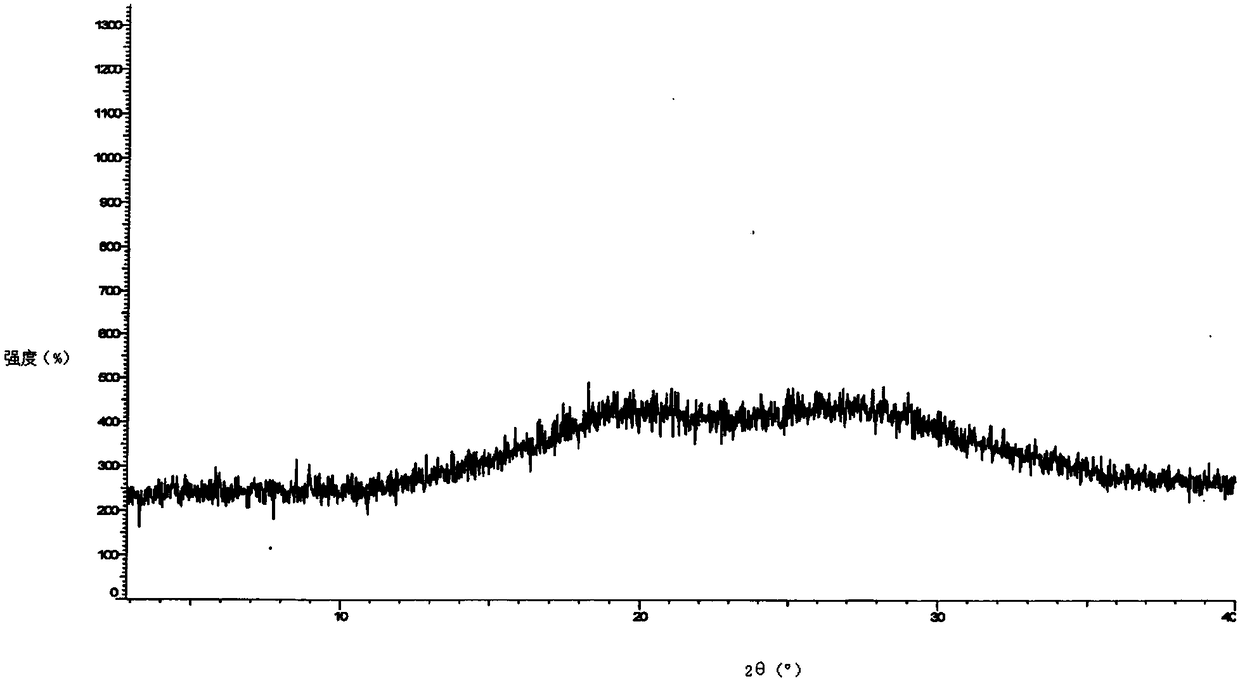
Preparation method of darunavir in amorphous form
The invention relates to the technical field of medical chemistry, in particular to a preparation method of darunavir in an amorphous form. The preparation method belongs to an anti-solvent method incrystal form preparation screening; although anti-solvents are already used to screen crystal form in the prior art, specific crystal forms of darunavir rather than the amorphous form like WO2013114382 are finally obtained. Different from the prior art, the preparation method has the advantages of being high in productivity and suitable for industrial production.
Owner:JIANGSU RUIKE MEDICAL SCI & TECH CO LTD
A synthetic method of a critical intermediate of darunavir
ActiveCN106083656ASimple post-processingMild reaction conditionsCarbamic acid derivatives preparationOrganic compound preparationCarbamateDarunavir+Ritonavir
A synthetic method of a critical intermediate of darunavir is disclosed. The critical intermediate which is tert-butyl 1S,2S-(1-benzyl-3-chloro-2-hydroxy propyl)carbamate is prepared by adopting tert-butyl 1S-(1-benzyl-3-chloro-2-carbonyl propyl)carbamate as a raw material through catalytic hydrogenation by using a chiral carbonyl reducing catalyst that is {N-[3-(Eta6-phenyl)propyl]-[(1S,2S)-1,2-diphenyl-1-4-methyl benzsulfamide (kN)-ethyl-2-amino-(kN)]}ruthenium (II). The method is simple in route and mild in reaction conditions. Through test verification, the yield is high and optical purity is high. After-treatment of tert-butyl 1S,2S-(1-benzyl-3-halogen-2-hydroxy propyl)carbamate after the reaction is finished is simple, and the chiral carbonyl reducing catalyst can be separated by filtering. A solvent is recovered and reutilized, and the catalyst can be recycled, and therefore the cost is low, an idea of green chemistry is met, and a three-waste discharge problem of the tert-butyl 1S,2S-(1-benzyl-3-halogen-2-hydroxy propyl)carbamate in the prior art is overcome, and the cost is reduced.
Owner:LIANYUNGANG DUXIANG CHEM
Oral preparation containing darunavir
The invention discloses oral preparation containing darunavir and particularly relates to oral preparation containing the darunavir, tipranavir and ritonavir. Besides, the oral preparation can select one of a binder, a lubricant and a dispersing agent or the mixture of the binder, the lubricant and the dispersing agent according to the type of the preparation. The oral preparation which is used for treating aids and is formed by combining different effective doses is prepared by adjusting the contents of the darunavir, the tipranavir and the ritonavir; in the preparation, the content of the darunavir is preferable 300-1,000mg, the content of the tipranavir is preferable 150-600mg, and the content of the ritonavir is preferable 100-400mg; in the preparation, the total weight of the darunavir, the tipranavir and the ritonavir is preferable 650-1,800mg; the proportion of the binder is preferable 5-15 percent of the total weight of a unit preparation, the proportion of the lubricant is preferable 0.5-1.5 percent of the total weight of the unit preparation, and the proportion of the dispersing agent is preferable 1.5-3.5 percent of the total weight of the unit preparation.
Owner:VIWIT PHARMA
Process for preparation of darunavir and darunavir ethanolate of fine particle size
The present invention provides a novel process for preparation of darunavir that involves reduction of [(1S,2R)-3-[[(4-nitrophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]carbamic acid (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester, of formula (5). The present invention also provides darunavir ethanolate of particle size wherein d0.9 is less than 130 μm, d0.5 is less than 30 μm, d0.1 is less than 10 μm and process for its preparation.
Owner:LUPIN LTD
Anti-Retroviral Combination
A pharmaceutical composition comprising a solid unit dosage form comprising:(i) ritonavir or a pharmaceutically acceptable salt and ester thereof;(ii) darunavir or a pharmaceutically acceptable salt and ester thereof.
Owner:CIPLA LTD
Anti-retroviral combination
ActiveUS9339470B2Easy to manufactureAntiviralsPharmaceutical non-active ingredientsDarunavir+RitonavirDosage form
Owner:CIPLA LTD
A Multi-Class Anti-Retroviral Composition
The present invention is related to an anti-retroviral composition. In particular, the present invention relates to a solid oral composition comprising combination of multi-class drugs particularly darunavir, dolutegravir and cobicistat and process for preparing the same.
Owner:HETERO LABS LTD
Process for synthesis of syn azido epoxide and its use as intermediate for the synthesis of amprenavir & saquinavir
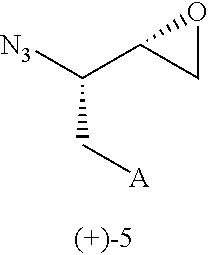
Disclosed herein is a novel route of synthesis of syn azide epoxide of formula 5, which is used as a common intermediate for asymmetric synthesis of HIV protease inhibitors such as Amprenavir, Fosamprenavir, Saquinavir and formal synthesis of Darunavir and Palinavir obtained by Cobalt-catalyzed hydrolytic kinetic resolution of racemic anti-(2SR,3SR)-3-azido-4-phenyl-1,2-epoxybutane (azido-epoxide).
Owner:COUNCIL OF SCI & IND RES
Novel process to prepare intermediates of HIV-protease inhibitors thereof
InactiveUS20160075643A1Conveniently to industrial scaleHigh yieldOrganic compound preparationSulfonic acid amide preparationDarunavir+RitonavirProteinase activity
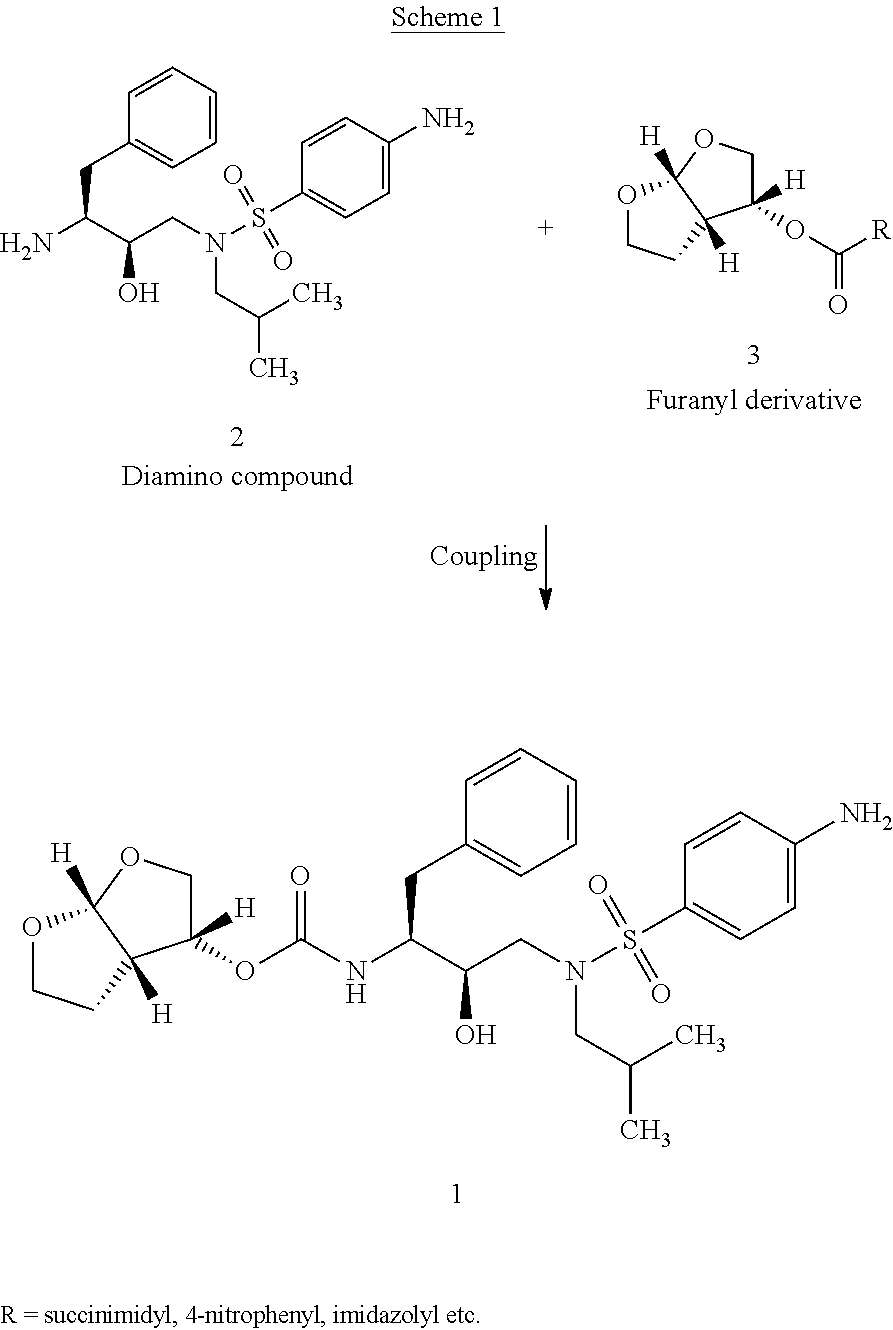
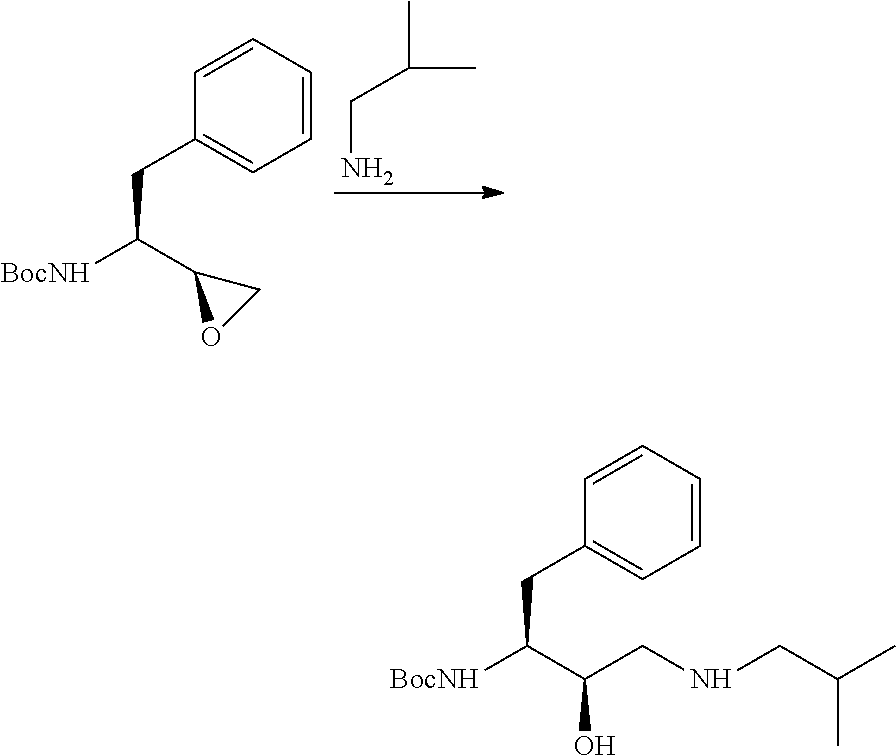
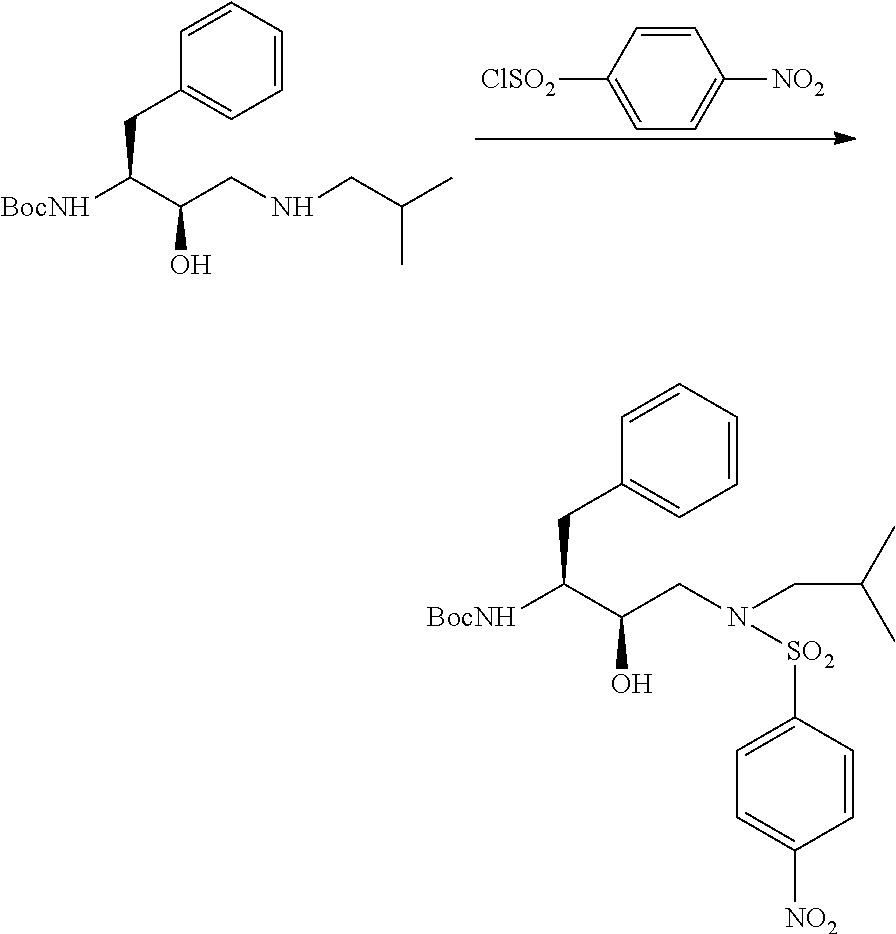
The present invention relates to an industrially feasible and economically viable process for the preparation of (1S,2R)-3-[[4-aminophenyl)-sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenyl-methyl)propyl]amine of formula I and its salt thereof and optionally converting it to HIV-protease inhibitors like Darunavir, Amprenavir or its prodrug Fosamprenavir.
Owner:ZCL CHEM
Novel process for preparation of darunavir and darunavir ethanolate of fine particle size
InactiveUS20140066638A1Organic compound preparationHydroxy compound preparationFuranDarunavir+Ritonavir
Owner:LUPIN LTD
Darunavir novel crystal form as well as preparation method and application thereof
InactiveCN108727401AEasy to compressImprove liquidityOrganic chemistry methodsAntiviralsFuranDarunavir+Ritonavir
The invention relates to a darunavir novel crystal form as well as a preparation method and application thereof, in particular to a novel crystal form of [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxyl-1-(phenyl methyl)propyl]-carbamic acid (3R, 3aS, 6aS)hexahydrofuran[2,3-b]furan-3-yl ester and a preparation method thereof. The crystal form is stable, is easy to store, andis better in mobility. Moreover, the preparation method is simple, can be industrially applied, and has a wide drug prospect.
Owner:YANCHENG DESANO PHARMA CO LTD
Process for preparing darunavir amorphous
ActiveUS10513527B2Simple processNo special equipment requiredOrganic chemistryAnti solventDarunavir+Ritonavir
The present invention relates to medical chemistry technique field, particularly relates to a preparation method for amorphous darunavir crystal,The present invention provides a preparation method for preparing amorphous form of darunavir using an anti-solvent method. It can be a different method compared to the evaporation and concentration process disclosed in WO2011048604. Although the same with WO2013114382, anti-solvent are used, the target crystal form are truly different. The crystal form of WO2013114382 is solvent-free darunavir crystal, while an amorphous form of darunavir in the present application.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Solvates of darunavir
ActiveUS9643976B2Simple, industrially applicable and economically viableOrganic chemistryAntiviralsDarunavir+RitonavirChemistry
Owner:AUROBINDO PHARMA LTD
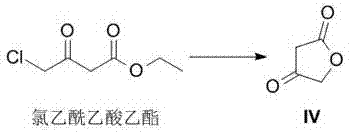
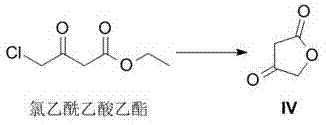
A kind of method for preparing darunavir intermediate
The invention discloses a method for preparing a darunavir intermediate. In the method, ethyl chloroacetoacetate is used as a raw material, and a compound of formula I, a key intermediate of darunavir, is obtained through four-step reactions. The method is reasonable in process, simple in operation, low in cost and high in yield, can well realize industrialization through the method, and improves production efficiency.
Owner:成都博腾药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com