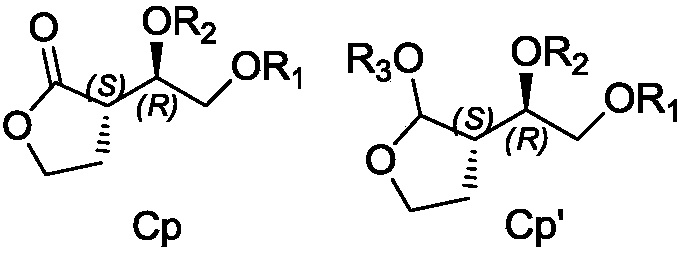
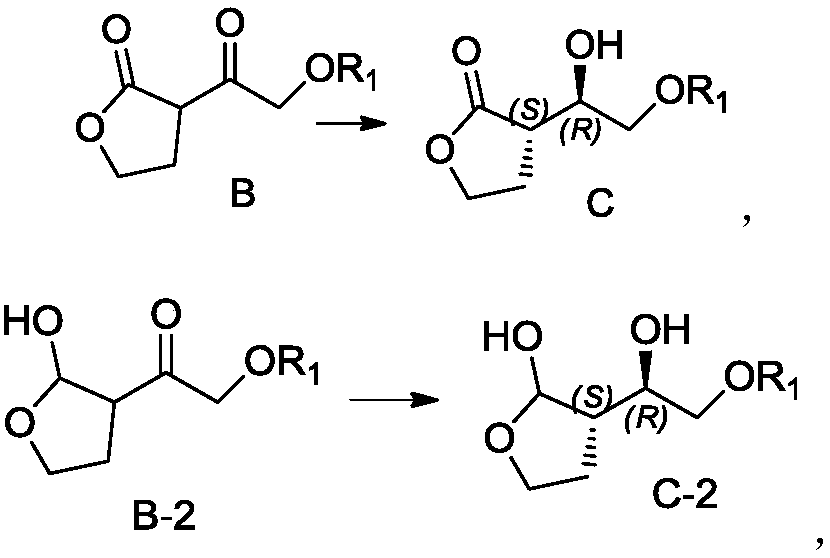
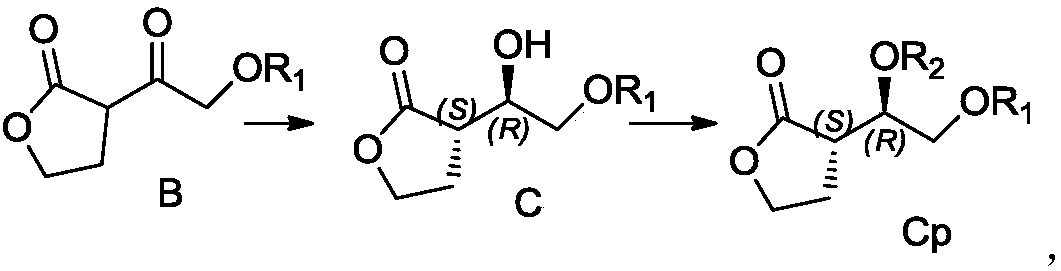
Preparation method for hexahydrofurofuranol derivative, intermediate of hexahydrofurofuranol derivative, and preparation method for intermediate
A technology of hexahydrofuran and body formula, which is applied in the field of preparation of hexahydrofuran and furan alcohol derivatives, can solve the problems that are not suitable for industrialization, and achieve the effect of low cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088]
[0089] Add the compound of formula A1, dichloromethane, into the reaction flask, cool down, weigh bromine, add dichloromethane to dilute, transfer the diluted bromine to the dropping funnel, add slowly dropwise, and control the internal temperature. After dropping, keep warm for reaction and control internal temperature. Add water, control the temperature, let it stand, and separate the liquid. The lower organic phase was put into another reaction flask, water was added, extracted and separated. The lower organic phase was put into another reaction flask, and 5% NaHCO was added 3 Aqueous solution extraction, liquid separation. The lower organic phase was put into another reaction flask, and the upper aqueous phase was combined and added with dichloromethane for extraction and liquid separation. Discard the aqueous phase and combine the organic phase, add water, extract, separate the liquid, discard the aqueous phase, and the lower organic phase, and place in a r...
Embodiment 2
[0091]
[0092] Put acetone, compound of formula A2 (X is bromine), benzoic acid into the reaction bottle, stir and cool down. Add triethylamine into the dropping tank, start to drop slowly, and control the internal temperature. After dropping, the temperature was raised to room temperature, and the reaction was stirred. After the reaction is finished, filter with suction. After the filter, transfer the filtrate to a distillation bottle for vacuum distillation. Control the temperature at 50-60° C. until the distillation bottle appears solid and becomes a paste, add ethyl acetate and steam. Add ethyl acetate to the distillation bottle and stir until it dissolves evenly, transfer the material liquid in the distillation bottle to the reaction flask, add saturated brine to the reaction flask for washing, let stand, separate the liquids, combine the water layers, and add ethyl acetate Extract, stand still, separate layers, discard the water layer, combine the organic layers, ad...
Embodiment 3
[0094] Preparation of Whole Cells of Genetically Engineered Bacteria with Aldehyde and Keto Reductase
[0095] Recombinant aldehyde and ketone reductase genetically engineered bacteria, the specific preparation method is: select the gene sequence of aldehyde and ketone reductase derived from Saccharomyceskudriavzevii, carry out artificial design, and synthesize the artificially designed sequence through the whole gene (entrusted by KingScript Biotechnology Co., Ltd. Synthetic), cloned into the Nde I and Xho I restriction sites of the expression vector pET28a, transformed host bacteria E.coli BL21 (DE3) competent cells; picked positive transformants and sequenced and identified them to obtain recombinant expression vectors; recombinant The expression vector is transferred into the E. col i BL21 (DE3) strain, and the recombinant aldehyde and ketone reductase genetic engineering bacteria capable of inducing the expression of the recombinant aldehyde and ketone reductase are obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com