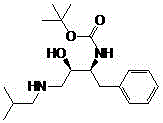
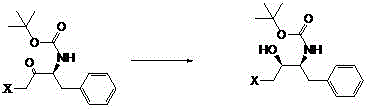
A synthetic method of a critical intermediate of darunavir
A synthetic method, the technology of darunavir, applied in the field of intermediates for the preparation of darunavir, can solve the problems of high discharge of three wastes and low chiral purity, and achieve the effects of low cost, high optical purity and simple route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, a synthesis method of a darunavir key intermediate: In a clean 150ml autoclave, put 20.0 grams of 1S, 2S-(1-benzyl-3-halo-2-carbonylpropyl) carbamic acid tert-butyl ester, 0.2 g of carbonyl reduction catalyst {N-[3-(η6-phenyl)propyl]-[(1S-2S)-1,2-diphenyl-1-4-methylbenzenesulfonamide (kN)-Ethyl-2-amino-(kN)]}ruthenium (II), add 100ml of methanol for hydrogen replacement 3 times, hydrogen pressure 0.5 MPa, stir, control the reaction at 30 ℃, react for 3h. Release the pressure and filter to obtain the filtrate and filter cake. The filter cake is rinsed with a small amount of methanol and applied mechanically again. The filtrate was distilled under reduced pressure, methanol was recovered, and applied mechanically again. The crude product was obtained with a yield of 95.2% and a content of 98.1%. MS(+1)=337.5.
Embodiment 2
[0034]Example 2, a synthesis method of a darunavir key intermediate: In a clean 150ml autoclave, put 20 grams of 1S, 2S-(1-benzyl-3-halo-2-carbonylpropyl) carbamic acid tert-butyl ester, 0.5 g of carbonyl reduction catalyst {N-[3-(η6-phenyl)propyl]-[(1S-2S)-1,2-diphenyl-1-4-methylbenzenesulfonamide (kN)-Ethyl-2-amino-(kN)]} ruthenium (II), add 100ml of methanol for hydrogen replacement 3 times, hydrogen pressure 0.5MPa, control the reaction at 30°C, and react for 3h. Release the pressure and filter to obtain the filtrate and filter cake. The filter cake is rinsed with a small amount of methanol and applied mechanically again. The filtrate was distilled under reduced pressure, methanol was recovered, and applied mechanically again. The crude product was obtained with a yield of 95.6% and a content of 98.3%. MS(+1)=337.94.
Embodiment 3
[0035] Example 3, a synthesis method of a darunavir key intermediate, put 20 grams of 1S, 2S-(1-benzyl-3-halo-2-carbonylpropyl) carbamic acid into a clean 150ml autoclave tert-butyl ester, 0.2 g of carbonyl reduction catalyst {N-[3-(η6-phenyl)propyl]-[(1S-2S)-1,2-diphenyl-1-4-methylbenzenesulfonamide (kN)-Ethyl-2-amino-(kN)]} ruthenium (II), add 100 ml of methanol for hydrogen replacement 3 times, hydrogen pressure 1.0 MPa, control the reaction at 30°C, and react for 3 hours. Release the pressure and filter to obtain the filtrate and filter cake. The filter cake is rinsed with a small amount of methanol and applied mechanically again. The filtrate was distilled under reduced pressure, methanol was recovered, and applied mechanically again. The crude product was obtained with a yield of 95.2% and a content of 97.5%. MS(+1)=337.94.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com