Darunavir midbody as well as preparation method and application thereof
An intermediate and reaction technology, applied in the field of drug synthesis, can solve the problems of uneconomical industrial scale production, low reaction yield, and difficulty in realization, and achieve the effects of low cost, simple operation, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
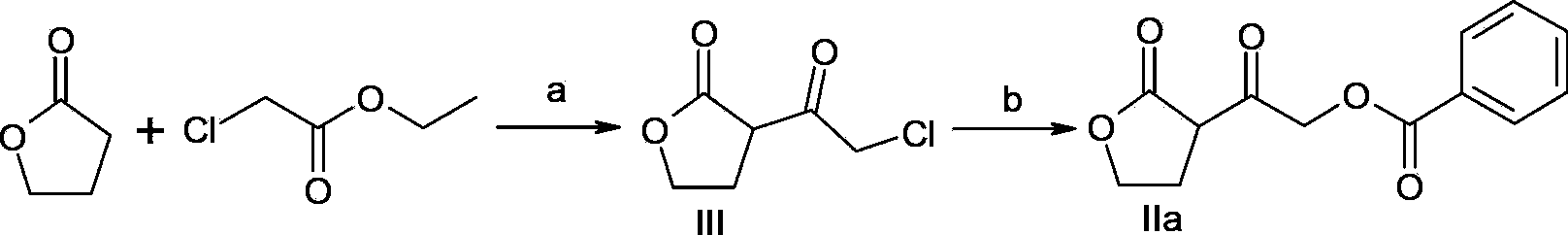
[0026] Embodiment 1: Preparation of intermediate of the present invention (compound of formula IIa)
[0027]
[0028] a) Add lithium hexamethyldisilazide (LiHMDS) (1.1L, 1mol / L) into the reaction flask under the protection of argon, cool the system to -78°C, add 1,4-butyrolactone dropwise (88.75g, 1.03mol) THF solution 250mL, after dropping, keep warm at -78°C and stir for 1 to 3 hours; add 250mL THF solution containing ethyl chloroacetate (125.05g, 1.02mol) dropwise, keep warm at -78°C after dropping Stir at ℃ for 1 to 3 hours; add 100mL of 4N hydrochloric acid aqueous solution, stand still, separate the liquids, extract the aqueous phase with ethyl acetate (100mL×3), combine the organic phases, wash with 100mL saturated brine, dry over anhydrous sodium sulfate, and filter. The filtrate was concentrated under reduced pressure and dried to obtain 156.07 g of a light yellow oil (compound of formula III), with a molar yield of 96% and an HPLC purity of 96.3%.
[0029] b) The...
Embodiment 2
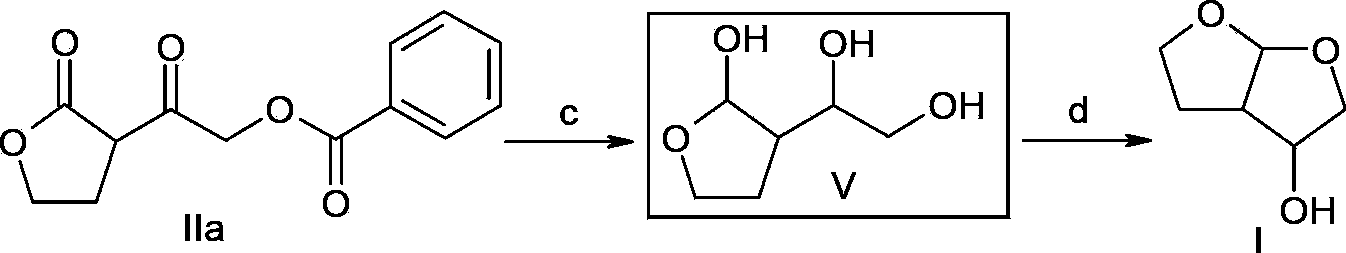
[0032] Embodiment 2: application above-mentioned intermediate preparation formula I compound
[0033]
[0034]c) Dissolve the compound of formula IIa (2.49g, 0.01mol) in 25mL of absolute ethanol, slowly add sodium borohydride (1.14g, 0.03mol) under ice-water bath, then react at room temperature for 0.5-4 hours, add 10m3L4N hydrochloric acid The aqueous solution was extracted with ethyl acetate (20mL×3), the organic layers were combined, the organic phase was washed with 20mL saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and dried to obtain a light yellow oil (compound of formula V ) 1.16g, directly used in the next step reaction.
[0035] d) Dissolve the compound of formula V (4.0g, 0.027mol) in 9mL of tetrahydrofuran, add methanesulfonic acid (0.34g, 0.0027mol) under ice-cooling, after the addition is complete, heat to 30-55°C, and keep stirring for 0.5 ~2 hours, cooled to room temperature, added trie...
Embodiment 3
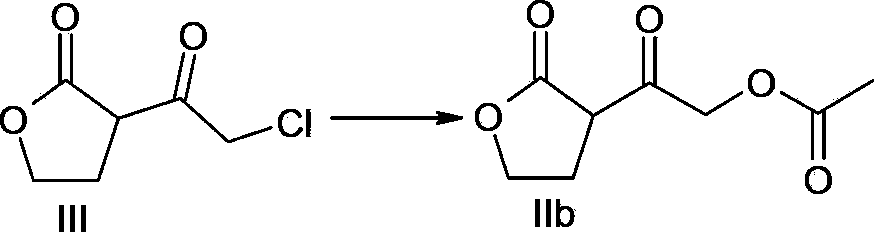
[0036] Embodiment 3: preparation intermediate of the present invention (compound of formula IIb)
[0037]
[0038] The compound of formula III (16.2g, 0.1mol) prepared according to the method in Example 1 was dissolved in 50mL of acetone, and acetic acid (6.6g, 0.11mol) was slowly added; triethylamine (11.1g, 0.11mol), the dropwise addition was completed, and the temperature was raised to reflux. When the reflux reaction was completed (about 2 to 5 hours), a large amount of white solids precipitated, filtered, and the filtrate was concentrated under reduced pressure to obtain a yellow solid. Add 30 mL of ethyl acetate and 20mL saturated sodium bicarbonate solution, separated, the aqueous phase was extracted with ethyl acetate (20mL×2), the organic phases were combined, washed with 20mL saturated brine, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and dried to obtain Pale yellow oil (compound of formula IIb) 16.3g, mola...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com