Darunavir novel crystal form as well as preparation method and application thereof
A crystal and amorphous technology, applied in the field of preparation of new crystal forms, can solve problems affecting bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention also provides a preparation method of D1-type darunavir crystals.
[0056] The preparation method of the crystal of the present invention, the preparation method comprises the following steps:
[0057] (1) Stir the amorphous darunavir crystals in a mixed solvent of alcohol and water for 5 to 15 hours at room temperature;
[0058] (2) Filter, collect crystals, and dry.
[0059] In another preferred example, the alcohol in step (1) is selected from one or more of methanol, ethanol, and isopropanol; the weight ratio of amorphous darunavir, alcohol, and water is 1:0.25-3.0 : 4.0~10.0; the drying temperature described in the step (2) is 30~45° C.
[0060] Pharmaceutical compositions and applications
[0061] The present invention also provides a pharmaceutical composition, which contains active ingredients in a safe and effective dose range, and a pharmaceutically acceptable carrier.
[0062] The "active ingredient" mentioned in the present inventio...
Embodiment 1
[0089] Weigh 100g of amorphous darunavir, add it to 1000ml of methanol and water mixed solvent (methanol content 5%), stir at room temperature for 12 hours, filter, and dry at 35-40°C for 12-16 hours to obtain 95.8g of solid .
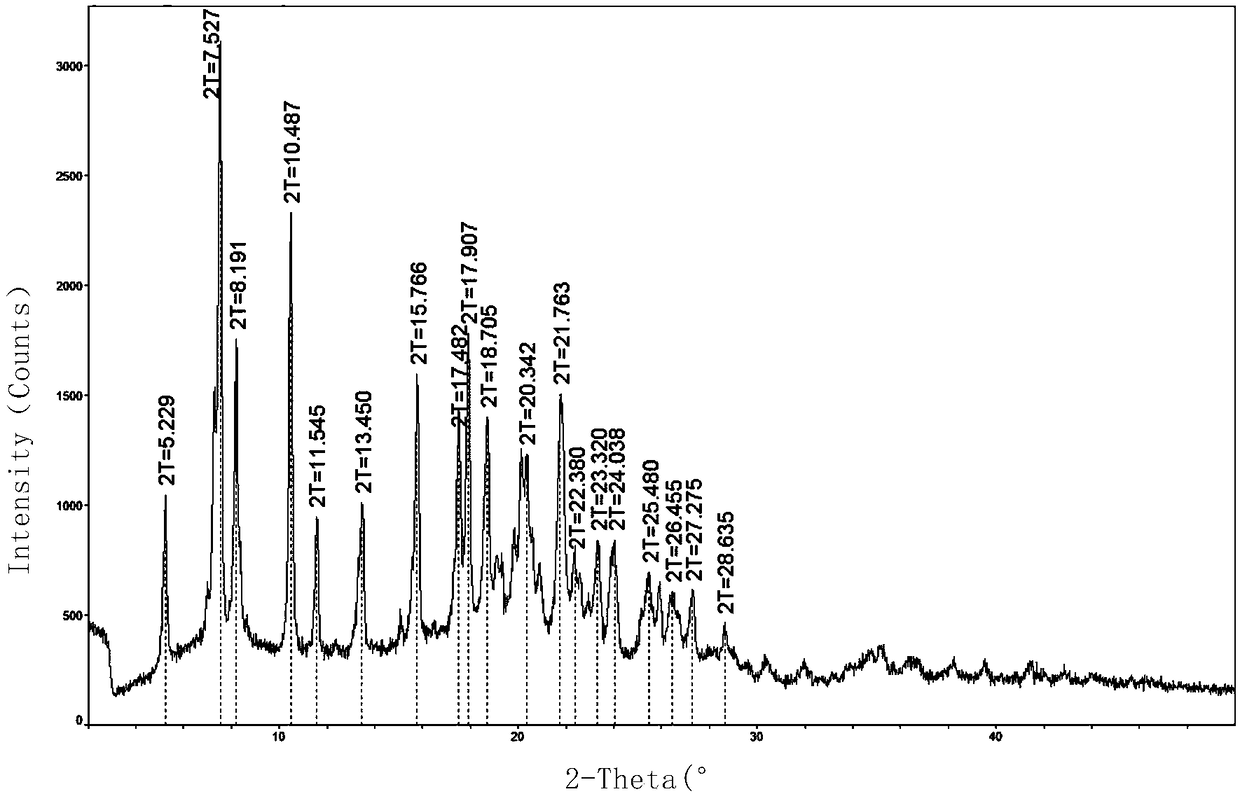
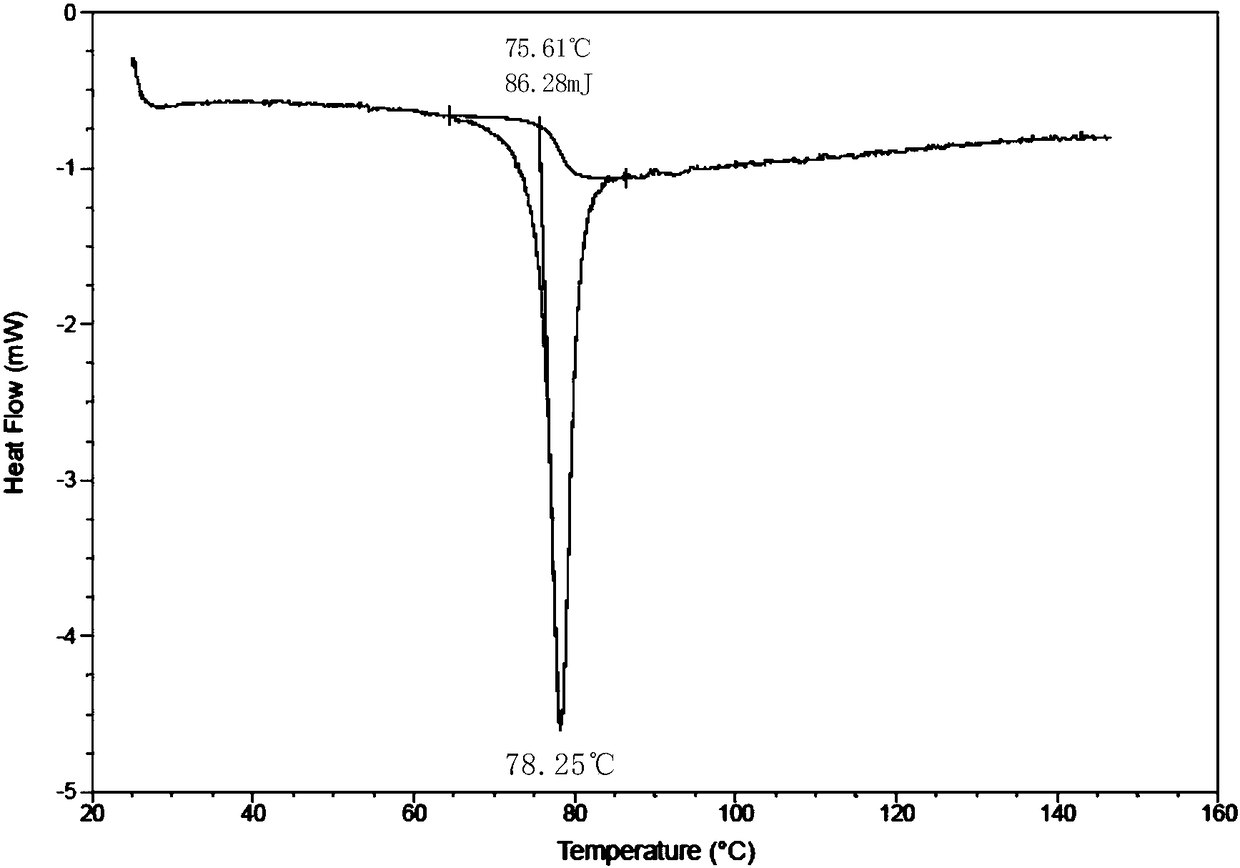
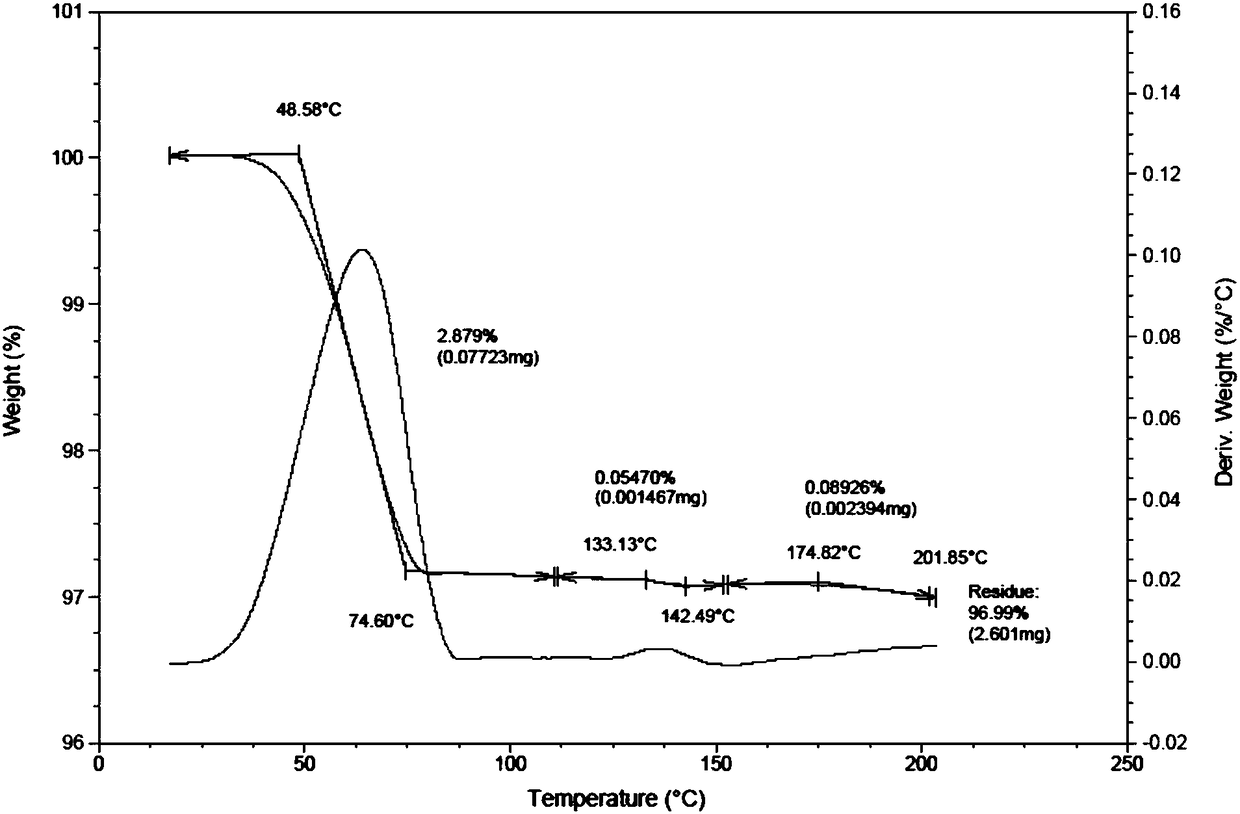
[0090] Result: the obtained solid, i.e. the powder X-ray diffraction pattern of crystal form D1 is as follows figure 1 Characterized, the differential thermal scanning spectrum is shown in 2, and its TGA and DSC combined spectrum is shown in image 3 As shown, the total water loss can be seen intuitively.
[0091] The moisture content in the crystal was determined to be 2.9% by KF moisture method.
Embodiment 2
[0093] Take by weighing 100g darunavir ethanol compound, join in the 500ml dichloromethane solution, after dissolving, concentrate to get foamy solid (being Darunavir amorphous), join in the mixed solvent of 800ml methanol and water ( methanol content 10%), stirred at room temperature for 10 hours, filtered, and dried at 35-40° C. for 12-16 hours to obtain 89.2 g of solid.
[0094] Results: The XRPD, DSC and TGA spectra of the obtained solid were basically consistent with the crystal form D1.
[0095] The moisture content in the crystal was determined to be 3.0% by KF moisture method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com