Method for preparing Darunavir intermediate
A technology of Chinese formula and compound, applied in the field of preparation of AIDS drug darunavir intermediate, which can solve the problems of high raw material cost and poor atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Under nitrogen protection, add 270 mL of 1.6 mol / L n-butyl lithium n-hexane solution to a 500 mL flask, cool to -78°C, and add 34.4 g (2.0 eq) 1,4-butyrolactone dropwise. After the dripping is completed, heat and stir for 2 hours, and then drip 31.2g (1.0eq, 0.2mol) 1,4-dioxaspiro[4,5]dec-2-one (compound of formula IV) in tetrahydrofuran. After the reaction was kept for 16 hours, the reaction was quenched with saturated ammonium chloride solution, the temperature was raised to room temperature, the layers were separated, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, and the product was concentrated after concentration, with a yield of 38%.
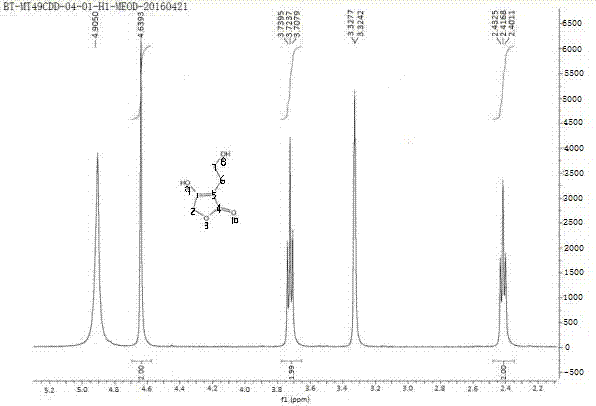
[0047] 1 H NMR (CD 3 OD, 400MHz):δ4.64 (s, 2H), 3.72(t, J=6.3Hz, 2H), 2.42(t, J=6.3Hz, 2H)
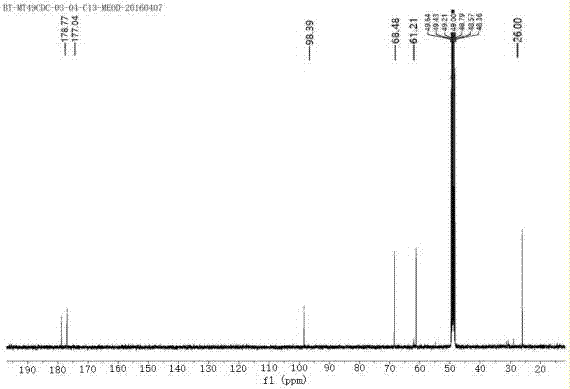
[0048] 13 C NMR(CD 3 OD, 400MHz): δ178.77, 177.02, 98.39, 68.48, 61.21, 26.00
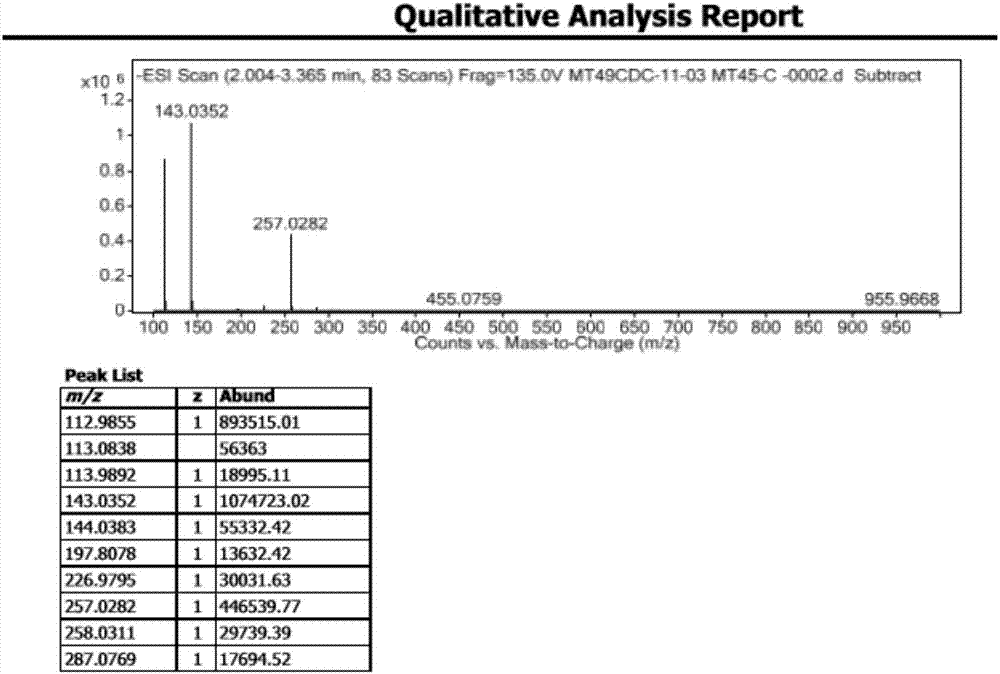
[0049] HRMS: M-1=143.0352
[0050] Melting point: 115-117°C.
Embodiment 2
[0052] Under the protection of nitrogen, add 160mL of 2.0mol / L lithium diisopropylammonium solution to a 250mL flask, cool to -78°C, and add 21.5g (2.5eq) 1,4-butyrolactone dropwise. After the dripping is completed, heat and stir for 2 hours, and then drip 15.6g (1.0eq, 0.1mol) 1,4-dioxaspiro[4,5]dec-2-one (compound of formula IV) in tetrahydrofuran. After the reaction was kept for 8 hours, the reaction was quenched with saturated ammonium chloride solution, the temperature was raised to room temperature, the layers were separated, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, and the product was concentrated after concentration, with a yield of 40%.
Embodiment 3
[0054] Under nitrogen protection, add 220mL 1.0mol / L lithium bis(trimethylsilyl)amide (LHMDS) solution to a 250mL flask, cool to -78℃, and add 17.2g (2.0eq) 1,4-butane dropwise ester. After the dripping is completed, heat and stir for 2 hours, and then drip 15.6g (1.0eq, 0.1mol) 1,4-dioxaspiro[4,5]dec-2-one (compound of formula IV) in tetrahydrofuran. After the reaction is kept for 12 hours, the reaction is quenched with saturated ammonium chloride solution, the temperature is raised to room temperature, the layers are separated, the aqueous phase is extracted with ethyl acetate, the organic phases are combined, and the product is obtained after concentration with a yield of 35%.
[0055] Synthesis of formula II compound
[0056] Example one
[0057] 5.04 g (0.035 mol) of compound III was added to a 100 mL autoclave, 50 mL of methanol was added, and the mixture was stirred to dissolve. Use high-purity nitrogen to change the air in the kettle three times, and then add 1.4g (0.05eq)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com