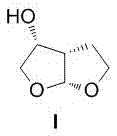
A method of preparing a darunavir intermediate
A technology of compounds and catalysts, applied in the field of medicine, can solve the problems of poor atomic economy and high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
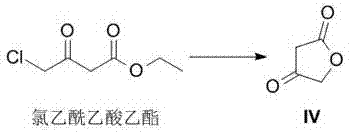
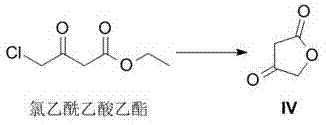
[0037] Synthesis of Example 1 Compound IV
[0038]
[0039] Add 45g (3eq) of potassium acetate and 120mL of acetic acid into a three-necked flask, and then add 25g (0.3mol, 1eq) of ethyl chloroacetoacetate; heat the above mixture to 80-90°C for 12h. After the reaction, cool to room temperature, filter, and collect the filtrate. Add 200mL of 10% hydrochloric acid solution to the filtrate and react at 15-20°C for 48 hours. Most of the acetic acid was distilled off under reduced pressure, and the remaining solution was extracted three times with 600 mL of ethyl acetate, the extracts were combined, and the solvent was distilled off to obtain compound IV, weight 13.5 g, yield 45%.
Embodiment 2
[0040] Synthesis of Example 2 Compound III
[0041]
[0042] Add 25g (0.25mol, 1eq) of compound IV, 69g (2.0eq) of potassium carbonate, 2.6g (0.05eq) of potassium iodide into the reaction flask, and add 200mL of N,N-dimethylformamide. Heat the mixture to 40-50°C for 30 minutes, then add 30g (1.5eq) of chloroethanol dropwise. After the addition, keep warm at 40-50°C for 24 hours. Filtrate, collect the filtrate, distill under reduced pressure, add 100mL water and 100mL ethyl acetate to the distillation residue, stir for 30min, separate after standing, extract the aqueous phase with 2×100mL ethyl acetate, combine the organic layers, and the organic phases are sequentially Washed with 60mL saturated saline and 60mL distilled water, separated after standing, dried the organic phase with anhydrous magnesium sulfate for 2 hours, filtered, and concentrated the filtrate to obtain compound III, weighing 27g, with a yield of 75%.
Embodiment 3
[0043] Synthesis of Example 3 Compound III
[0044] Add 20g (0.20mol, 1eq) of compound IV, 10.8g (1.1eq) of sodium methoxide, 1.5g (0.05eq) of potassium iodide into the reaction flask, and add 100mL of tetrahydrofuran. The mixture was heated to 20-30°C for 30 minutes, then 25g (1.5eq) of chloroethanol was added dropwise, and the temperature was raised to 40-50°C for 24 hours. Adjust the pH of the reaction solution to 4-5 with 2N dilute hydrochloric acid, separate the organic layer, extract the aqueous layer with 200 mL of ethyl acetate, combine the organic phases and dry over anhydrous magnesium sulfate for 2 hours, filter, and concentrate the filtrate to obtain compound III, weighing 30 g , The yield is 83.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com