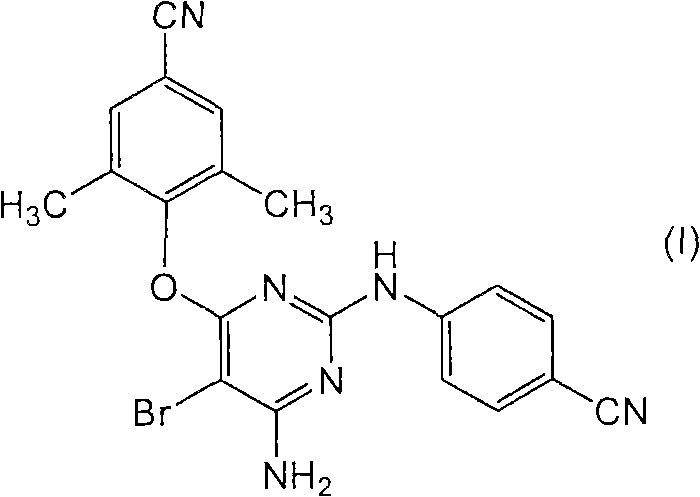
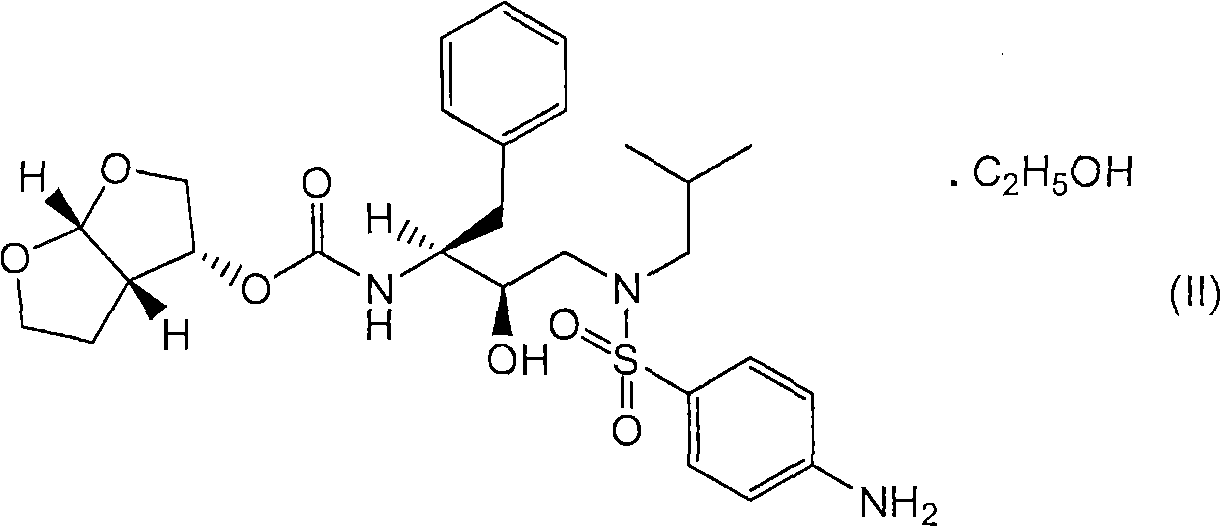
Combination formulations comprising darunavir and etravirine
A technology for dosage forms and oral preparations, which can be used in medical preparations containing active ingredients, pill delivery, antiviral agents, etc., and can solve problems such as physical difficulties, exceeding the convenient size limit, and large size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] 1) Preparation of spray-dried powder
[0106] The feed mixture for the spray-dried formulation was prepared by dissolving TMC125 and polymer in a solvent and adding microcrystalline cellulose. The polymer types, solvents and amounts of components used are listed under feed mixture (iv) mentioned above. The feed mixture was then passed through a high pressure nozzle in co-current mode into an SD-12.5-N, closed loop spray drying chamber. The resulting solid pharmaceutical composition is collected from the cyclone and post-vacuum dried at elevated temperature to reduce residual solvent levels. The dry powder is sieved, and the powder sieve with a particle size between 45-100 microns is retained.
[0107] 2) Preparation of Combination Tablets
[0108] Table 1: Composition of Combination Tablets
[0109] ingredient name
[0110] ingredient name
[0111] Spray-dried TMC125 in HPMC was mixed with microcrystalline cellulose (MCC), croscarmellose sodi...
Embodiment 2
[0119] 1) Preparation of spray-dried powders
[0120] The preparation of spray-dried TMC125 was carried out as described in Example 1.
[0121] 2) Preparation for rolling ( roller compaction ) bulk mixture
[0122] Table 4
[0123] Component mg / tablet
[0124] The ingredients were hand passed through a 1 mm stainless steel screen and then mixed in a 100 l Gallay tumble mixer at 10 rpm for 10 minutes. In Gerteis Polygran TM Roller compaction on 250 / 100 / 3 roller compactor.
[0125] Table 5: Roller compaction settings
[0126]
[0127] Table 6: Results of rolled material
[0128]
[0129] Table 7: Final Blending and Tabletting
[0130] Material mg / tablet
[0131] Except for magnesium stearate, all ingredients were passed through a 1mm stainless steel sieve, and then in 100 lGallay TM Mix for 10 minutes at 10 rpm in a tumble blender. The sieved magnesium stearate was then added to the mixture and mixed at the same speed for an a...
Embodiment 3
[0147] Example 3 : coating
[0148] Table 12: Dissolution Rates of Coated and Uncoated TMC125
[0149] Dissolution of TMC125 in Combination Tablets in Experiment 2
[0150] time (minutes)
5
10
15
20
30
45
60
120
180
210
1030daN uncoated
1266daN uncoated
1030daN coating
1266daN coating
31.67
16.77
21.99
46.35
14.60
27.45
37.79
57.37
20.96
37.80
46.23
65.95
28.70
46.96
52.07
75.10
42.77
62.47
60.11
80.93
52.56
80.84
67.31
84.83
61.74
89.56
72.54
93.07
76.12
98.21
83.87
96.29
81.10
99.61
88.91
98.15
88.90
99.34
91.43
[0151] Table 13: Dissolution Rates of Coated and Uncoated TMC114
[0152] Dissolution of TMC114 in Combination Tablets in Experiment 1
[0153] time (minutes)
5
10
15
20
30
45
60
120
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com