Medical intermediate and preparation method thereof
A technology for intermediates and medicines, applied in the field of medicine synthesis, can solve the problems of uneconomical industrial scale production, low reaction yield, difficult realization, etc., and achieve the effects of cheap raw materials, simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
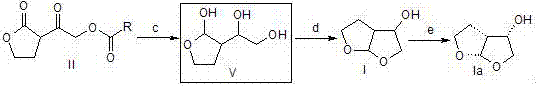
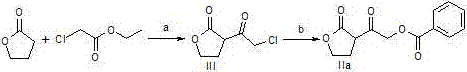
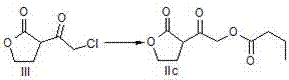
[0031] A preparation method of a pharmaceutical intermediate, firstly add 1.2~2.2eq strong base to the reaction bottle under the protection of argon, cool to -78°C, add dropwise a benign solvent of 1.05~1.5eq 1,4-butyrolactone, Incubate at -78°C for 2h; add 1eq ethyl chloroacetate benign solvent dropwise, and incubate at -78°C for 2h; add 6N hydrochloric acid aqueous solution to wash, and then wash with brine; (3) Concentrate the oil phase under reduced pressure to obtain a light yellow oil; then, Dissolve the above oil in acetone, slowly add 1.1~1.5eq aromatic acid or alkyl acid; slowly add 1.1~2.0eq triethylamine dropwise under ice bath, after the dropwise addition, raise the temperature to reflux; until a large amount of white solids are precipitated , filtered, and the filtrate was concentrated under reduced pressure to obtain a yellow solid; finally, ethyl acetate and saturated sodium bicarbonate solution were added, separated, extracted, washed, and dried to obtain the in...
Embodiment 1
[0035]
[0036] a) Add methyllithium (1.5L, 1.5mol) to the reaction flask under the protection of argon, cool the system to -78°C, add 1,4-butyrolactone (94.78g, 1.1mol) in 2 -Methyl tetrahydrofuran solution 300mL, after dripping, keep warm at -78°C for 2 hours; add 200mL of 2-methyltetrahydrofuran solution containing ethyl chloroacetate (122.60g, 1.0mol) dropwise, after dropwise keep warm, stir at -78°C for 2 hours Hours; 80 mL of 6N hydrochloric acid aqueous solution was added to stir and wash, washed with 150 mL of saturated brine, and the oil phase was concentrated under reduced pressure to obtain 156.88 g of a light yellow oil (compound of formula III), with a molar yield of 96.5% and an HPLC purity of 98.1%.
[0037]b) Dissolve the compound of formula III (15.61g, 0.096mol) in 40mL of acetone, slowly add benzoic acid (13.50g, 0.11mol); slowly add triethylamine (15.18g, 0.15mol) dropwise under ice-cooling, dropwise After completion, the temperature was raised to reflux...
Embodiment 2
[0039]
[0040] The compound of formula III (32.4g, 0.20mol) prepared according to the method in Example 1 was dissolved in 100mL acetone, and acetic acid (13.2g, 0.22mol) was slowly added; triethylamine (30.36g, 0.22mol) was slowly added dropwise under ice-cooling. 0.3mol), the dropwise addition was completed, the temperature was raised to the reflux reaction and then down to room temperature, a large amount of white solid was precipitated, filtered, and the filtrate was concentrated under reduced pressure to obtain a yellow solid, which was stirred and dissolved by adding 50mL ethyl acetate and 40mL saturated sodium bicarbonate solution, Stand for liquid separation, extract the aqueous phase with ethyl acetate (50mL×2), combine the organic phases, wash with 40mL saturated brine and concentrate the filtrate under reduced pressure to obtain 33.36g of a light yellow oil (compound of formula IIb), with a molar yield of 89.6% , HPLC purity is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com