Preparation method of darunavir in amorphous form
A darunavir and amorphous technology, applied in the field of medicinal chemistry, can solve the problems of extended production cycle, reduced production capacity, uneconomical and environmental protection, etc., and achieve the effect of short production cycle, large production capacity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
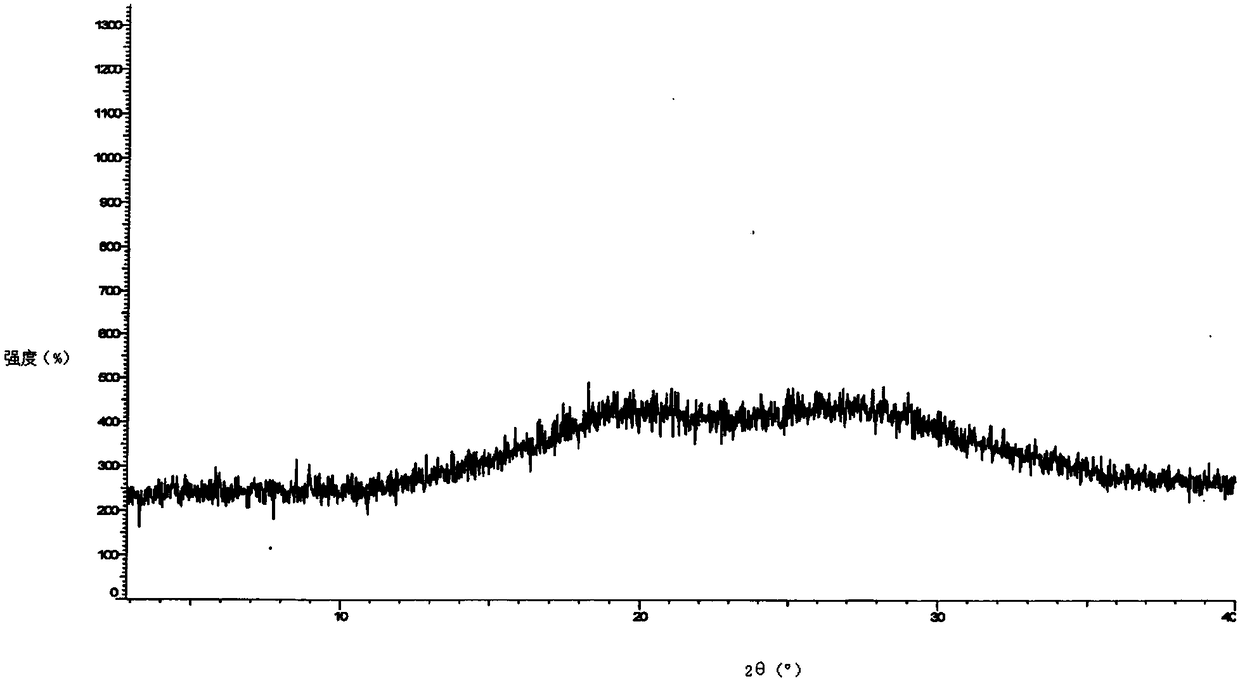
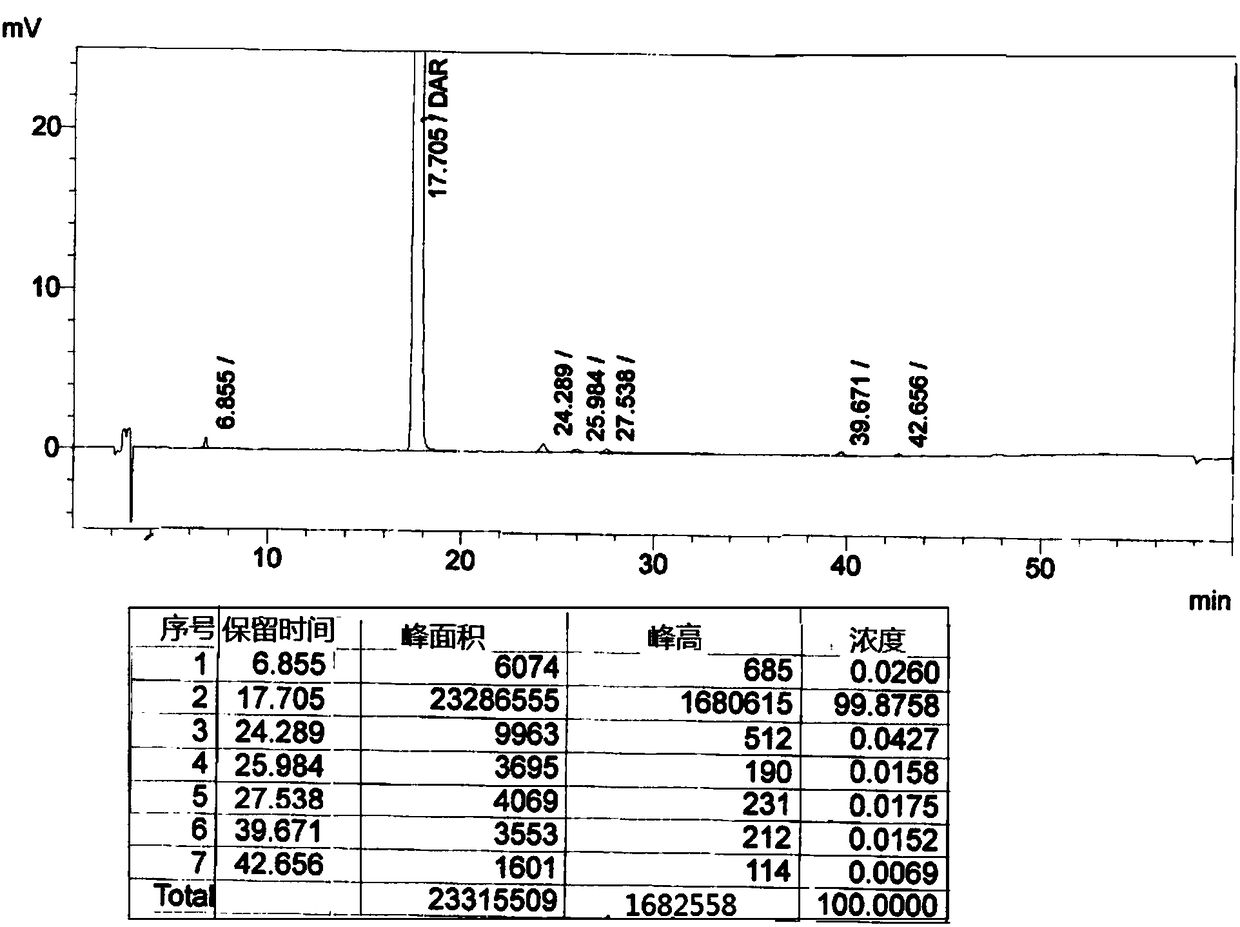
[0040] Put 20g of darunavir crude product and 20g of methanol into a clean and dry four-necked bottle, stir and heat up to 50°C-60°C to dissolve, then heat filter; add the solution dropwise to the aqueous sodium bicarbonate solution at 0°C-5°C , Continue to keep warm for 0.5-1 hour after adding. Suction filter and rinse with purified water. The wet product was dried to obtain the amorphous form of darunavir. Its XRPD as figure 1 As shown, its HPLC spectrum is as figure 2 shown.
Embodiment 2
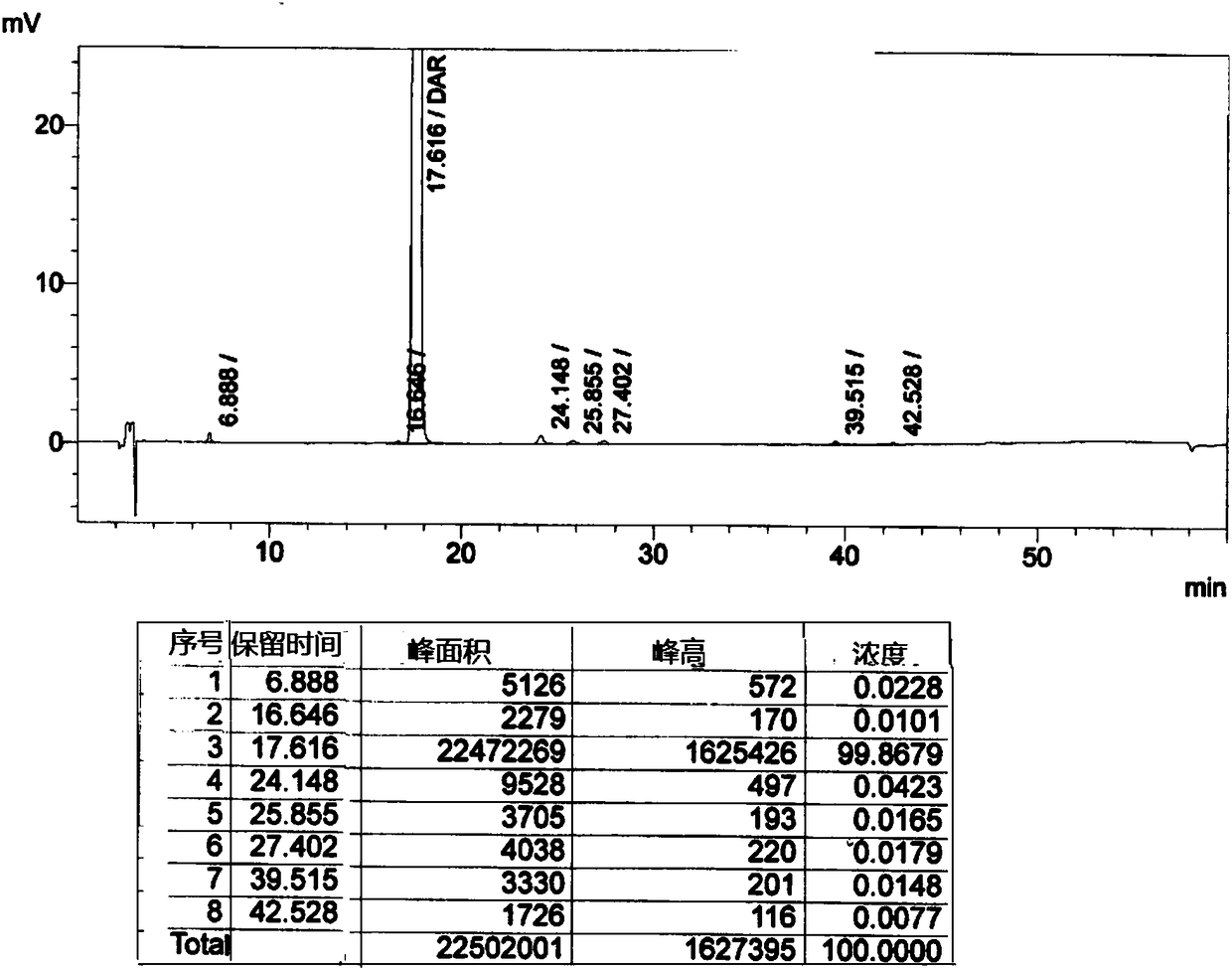
[0042] Put 20g of darunavir crude product and 40g of methanol into a clean and dry four-necked bottle, stir and heat up to 40°C-50°C to dissolve, then heat filter; add the solution dropwise to the aqueous sodium bicarbonate solution at 0°C-5°C , Continue to keep warm for 0.5-1 hour after adding. Suction filter and rinse with purified water. The wet product was dried to obtain the amorphous form of darunavir. Its XRPD pattern see image 3 .
Embodiment 3
[0044] Put 20g of darunavir crude product and 20g of methanol into a clean and dry four-necked bottle, stir and heat up to 50°C-60°C to dissolve, then heat filter; add the solution dropwise to water at 0°C-5°C, after the addition is complete Continue to keep warm for 0.5-1 hour. Suction filter and rinse with purified water. The wet product was dried to obtain the amorphous form of darunavir.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com