Synthetic method of dolutegravir key intermediate (R)-3-aminobutanol
A technology for the synthesis of aminobutanol, which is applied in the preparation of aminohydroxyl compounds, chemical instruments and methods, and the preparation of organic compounds, etc., which can solve the danger of using diazomethane, difficulty in obtaining R-alanine, and unsuitability for industrial production and other problems, to achieve the effect of improving optical purity, high product yield, and avoiding the use of dangerous raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
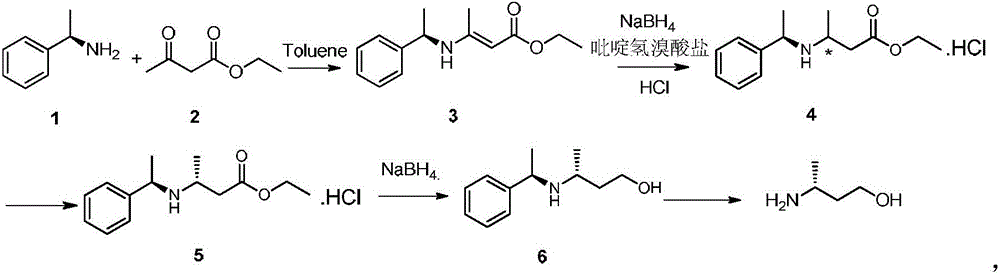
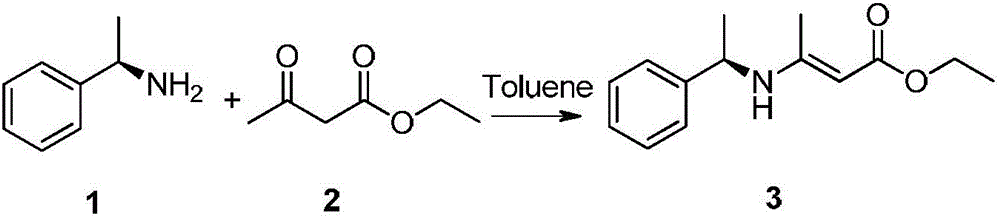
[0020] Add R-(+)-α-phenylethylamine (400g, 3.3mol) and ethyl acetoacetate (476g, 3.66mol) to the reaction flask equipped with water separator and reflux condenser, then add toluene (700mL) , pass in argon gas for inert gas protection, gradually heat up to reflux reaction, as the reaction progresses, pay attention to observe the amount of water separated from the water separator, when it remains stable and no longer increases, the reaction is basically over, and it takes about 3 -5h, the reaction liquid is light yellow, TLC monitors (ethyl acetate:n-hexane=1:5, v / v) the raw material reacts completely, finally evaporates the toluene under reduced pressure at 50°C, and then evaporates the unreacted under reduced pressure at 90°C Complete ethyl acetoacetate, finally (1R,2R) and (1R,2S)-3-(1'-methylbenzylamine)-2-butenoic acid ethyl ester (compound 3,720g, yield 93.6%) .
Embodiment 2
[0022]
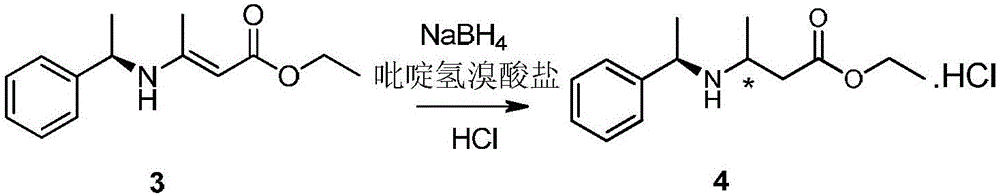
[0023] Double bond reduction: add NaBH to the reaction flask 4 (108g, 2.85mol) and methyl tert-butyl ether (MTBE, 2100mL), start stirring and cool down to -5°C, slowly drop compound 3 (300g, 1.29mol), control the reaction temperature at -5-0°C, After about 0.5h of dripping, keep the temperature and dropwise add 1500mL of methyl tert-butyl ether solution dissolved with 320g of pyridine hydrobromide, about 6h of dripping, after dripping, keep stirring for 0.5h, monitor by TLC (EA:PE=1: 5, v / v) The raw material reacts completely, then cool down to below -10°C and slowly add water dropwise. During the dripping process, gas is generated and the temperature rises. Control the dripping rhythm to keep the temperature not exceeding -10°C. After dripping water, add a certain amount of water. An appropriate amount of aqueous sodium hydroxide solution (mass concentration is 20%) makes the pH value of the reaction solution reach neutral, directly transfers to a separatory funne...
Embodiment 3
[0029]
[0030] Take compound 5 (52g, 0.19mol) and add it to a single-necked bottle, add water (100mL) and stir it into a paste, then slowly add potassium carbonate aqueous solution (mass concentration: 15%, 100g) dropwise, oily liquid gradually appears after the dropwise addition , then slowly add MTBE (100mL) to gradually dissolve the resulting oily liquid, and finally the reaction liquid is divided into two phases, the upper organic phase is separated, and anhydrous MgSO is added to the organic phase 4 (10 g) was dried and filtered with suction, and the filtrate was evaporated to remove the solvent to obtain free compound 5.
[0031] First add THF (90mL) into the reaction flask, pass through argon for inert gas protection, drop the temperature to 5°C and add NaBH 4 (21.5g, 0.51mol) and ZnCl 2 (34g, 0.25mol), then slowly add the free compound 5 dropwise, control the internal temperature at 5-10°C, drop it in about 2.5 hours, take a sample TLC to monitor the complete reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com