Preparation method of dolutegravir mother nucleus intermediate
A technology of dolutegravir and intermediates, which is applied in the field of organic synthetic medicinal chemistry, can solve the problems of low yield of intermediates, difficult industrialization, and large pollution, and achieve the effects of high selectivity, high safety, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
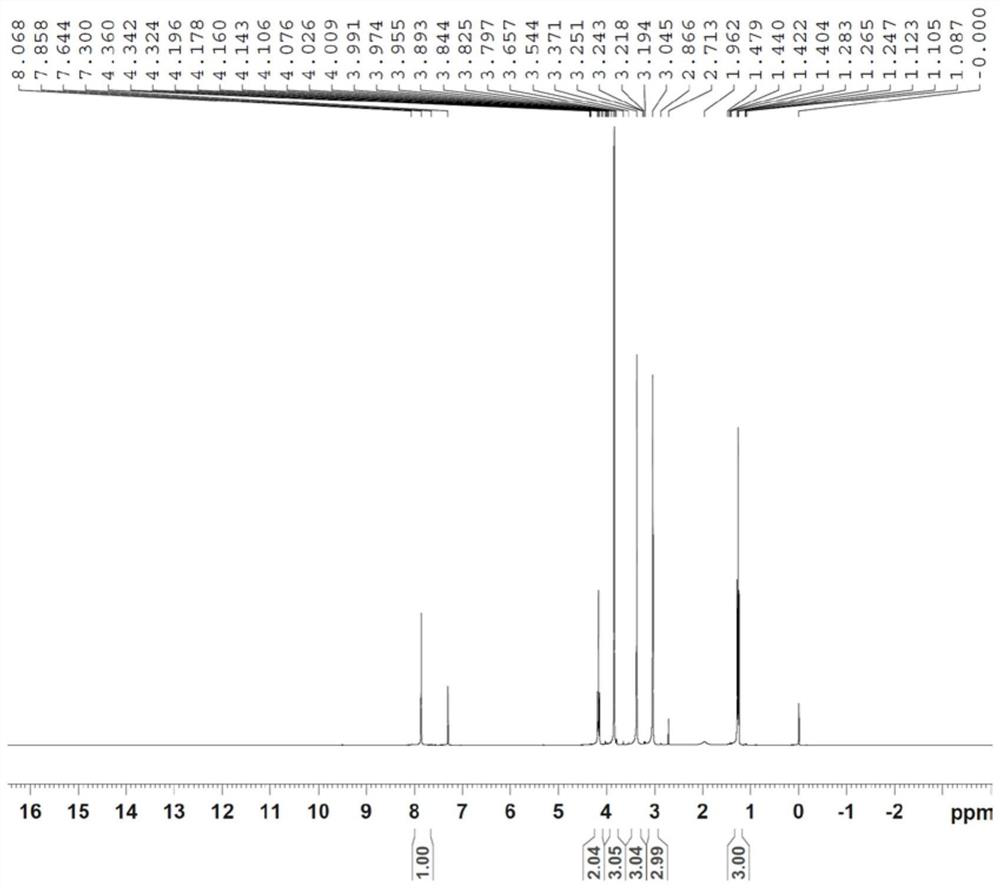
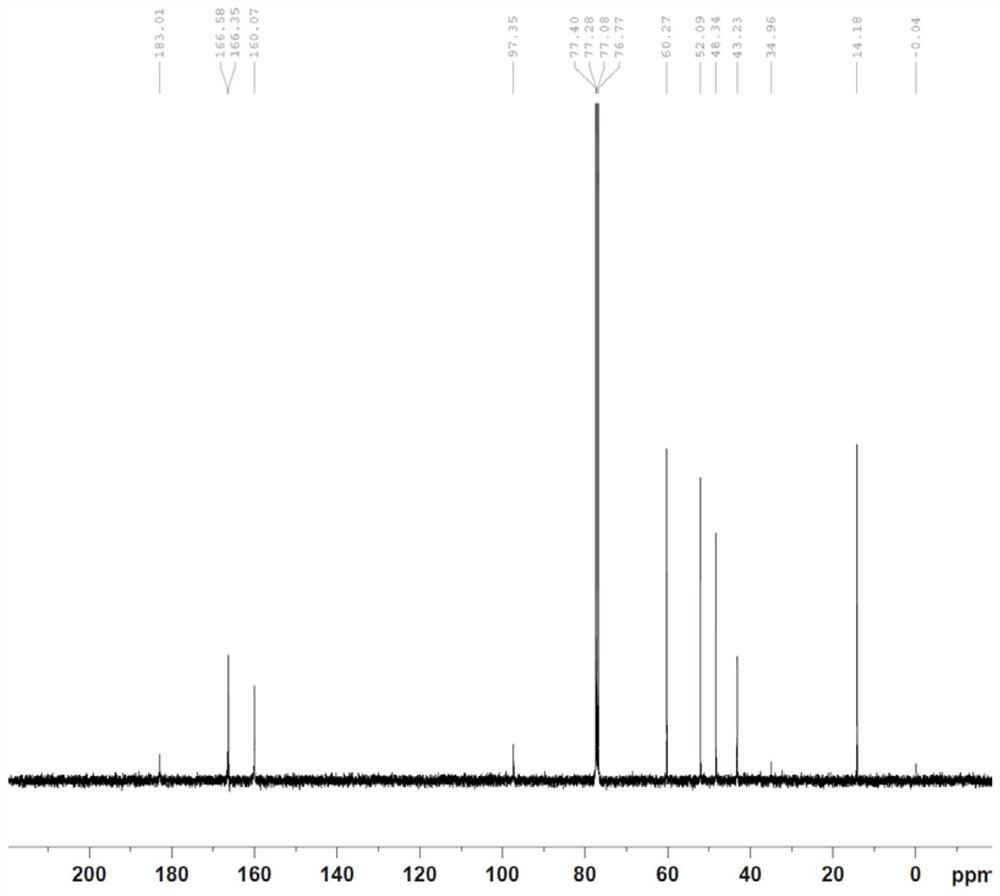
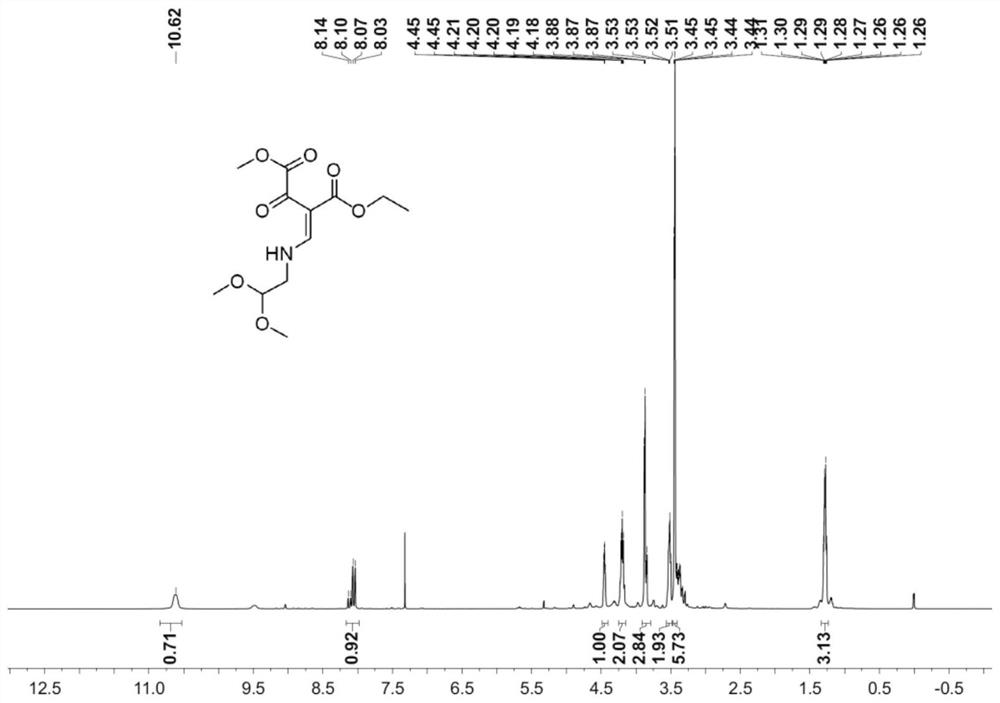
[0040] 1. Preparation of Compound P3
[0041]
[0042] In the following examples, N,N-ethyl dimethacrylate, N,N-ethyl dimethacrylate, dimethyl oxalate monoacyl chloride, and pyridine were all purchased from McLean Reagent Company.
Embodiment 11
[0044] Add 143g of N,N-ethyl dimethacrylate, 500g of dichloromethane, and 95g of pyridine into a 2L four-neck flask, stir well, cool to -15°C, slowly add 122g of dimethyl oxalate monoacyl chloride dropwise, and control the internal temperature When the temperature is lower than -5°C, keep stirring for 2 hours after adding, and the raw materials basically disappear by pointing the plate, slowly add aqueous sodium bicarbonate solution, adjust the pH to weak alkaline, separate the liquids, extract the aqueous phase again with dichloromethane, and combine the organic phases, 100g Washed with water, concentrated dichloromethane, refined with tertiary methyl ether to obtain 218g of pure product, with a yield of 95% and a purity of 99.5%.
Embodiment 12
[0045] Embodiment 1.2 (alkali and P2 equivalent molar ratio 3: 1)
[0046] Add 143g of N,N-ethyl dimethacrylate, 500g of dichloromethane, and 237g of pyridine into a 2L four-neck flask, stir evenly, cool to -15°C, slowly add 122g of dimethyl oxalate monoacyl chloride dropwise, and control the internal temperature When the temperature is lower than -5°C, keep stirring for 2 hours after adding, and the raw materials basically disappear by pointing the plate, slowly add aqueous sodium bicarbonate solution, adjust the pH to weak alkaline, separate the liquids, extract the aqueous phase again with dichloromethane, and combine the organic phases, 100g Washed with water, concentrated dichloromethane, refined with tertiary methyl ether to obtain 210 g of pure product, with a yield of 92% and a purity of 99.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com