Preparation method of (R)-3-aminobutanol
A technology of aminobutanol and amino, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of long reaction route, low overall yield, harsh process operation, etc., and achieve the effect of short reaction route, high optical purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
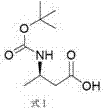
[0025] The present invention provides a preparation method of dolutegravir intermediate (R)-3-aminobutanol, which uses 3-(Boc-amino)butyric acid as the starting material and undergoes chiral resolution to obtain the compound of formula I;
[0026]
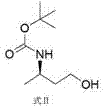
[0027] Then through sodium borohydride and Lewis acid reduction to obtain the compound of formula II;
[0028]
[0029] Finally, the amino group is deprotected to obtain (R)-3-aminobutanol.
[0030] The method of the present invention is described in further detail below with examples.
Embodiment 1
[0032] Dissolve 20.3 g of 3-(Boc-amino)butyric acid in 40.6 mL of methanol, stir and heat to 40-50°C, slowly add 9.1 g of S-phenylethylamine in 10.2 mL of methanol solution dropwise at this temperature, and dropwise, Insulate and stir for 20-30 min, slowly cool to 10-15°C, suction filter, and dry to obtain 14 g of carboxylate; then add 14 g of carboxylate into 28 mL of ethanol, heat to 70-75°C, and keep warm for 15-20 min, slowly lowered to 10-15°C, suction filtered, and dried to obtain 13 g of carboxylate. Repeat this operation twice to obtain 12.2 g of carboxylate with an ee value of 99.7%; finally add 12.2 g of carboxylate to 36 mL In water, adjust the pH to 1-2 with concentrated hydrochloric acid, add 30 mL of ethyl acetate to extract twice, combine the organic phases and concentrate until there is no distillate to obtain 7.6 g of the compound of formula I.
Embodiment 2
[0034] Dissolve 30 g of 3-(Boc-amino)butyric acid in 60 mL of ethanol, stir and heat to 70-75°C, slowly add 14 g of S-phenylethylamine in 15 mL of ethanol solution dropwise at this temperature, and dropwise, Insulate and stir for 20-30 min, slowly cool to 10-15°C, suction filter, and dry to obtain 21.6 g of carboxylate; then add 21.6 g of carboxylate into 43.2 mL of ethanol, heat to 70-75°C, and keep warm for 15-20 min, slowly lowered to 10-15°C, filtered with suction, and dried to obtain 20 g of carboxylate. Repeat this operation twice to obtain 18.8 g of carboxylate with an ee value of 99.6%; finally add 18.8 g of carboxylate to 55 mL In water, adjust the pH to 1-2 with concentrated hydrochloric acid, add 50 mL of ethyl acetate to extract twice, combine the organic phases and concentrate until there is no distillate, and obtain 11.7 g of the compound of formula I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com