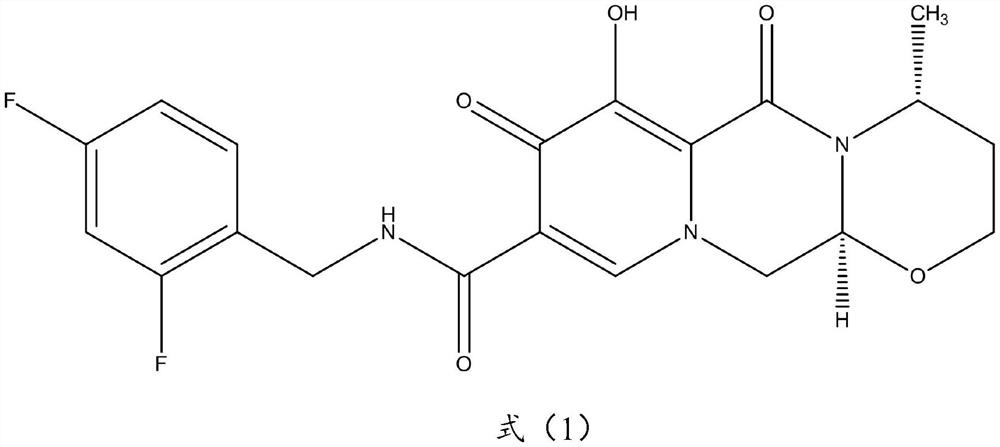
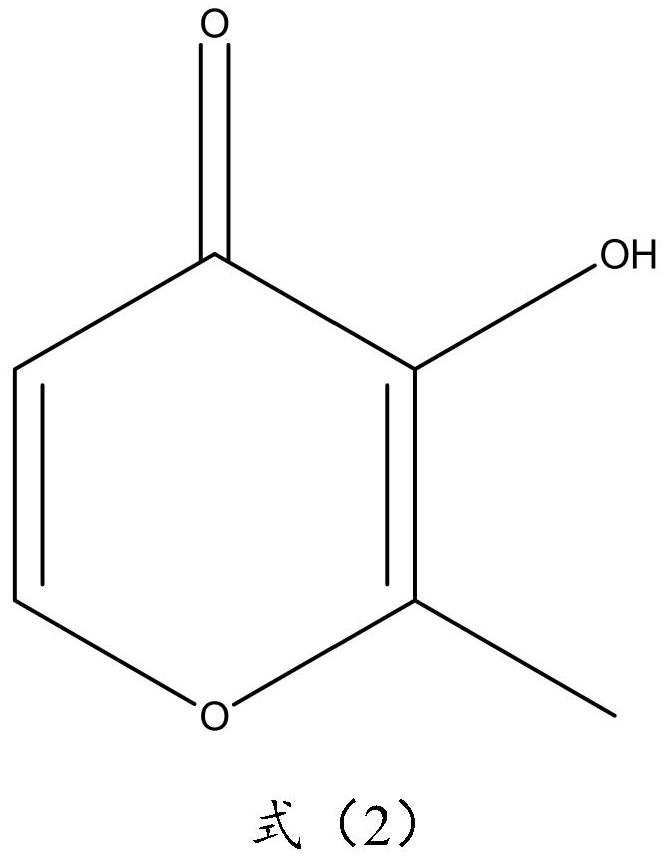
A kind of synthetic method of dolutegravir intermediate and its related substance detection method
A technology of dolutegravir and related substances, applied in the field of medicinal chemistry, can solve the problems of cumbersome steps, multiple detection methods, and long synthesis routes, and achieve the effects of simple analysis methods, simple reaction conditions, and universal conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
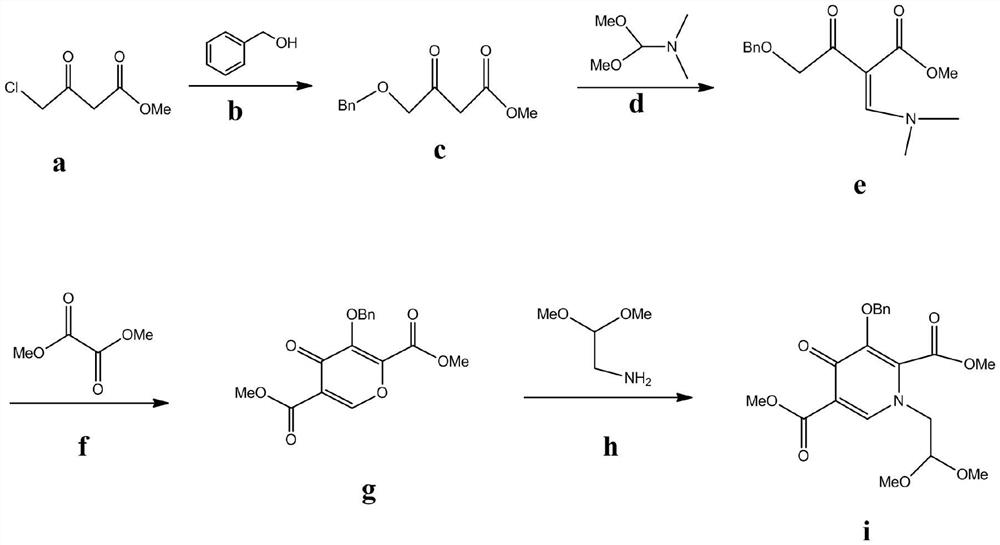
Embodiment 1
[0031] Synthesis of 4-(Benzyloxy)-3-oxobutyric acid methyl ester
[0032] Add 50mol methyl 4-chloroacetoacetate, 60mol benzyl alcohol, 20mol cesium carbonate and 500ml toluene into the reaction flask, put the reaction flask into an oil bath, heat to 110°C, reflux for 12h, after the reaction is completed, cool to room temperature, Filtration, rotary evaporation to remove the toluene solvent, and then use column chromatography to separate the crude product, the eluent is petroleum ether:dichloromethane=10:3, thereby obtaining 4-(benzyloxy)-3-oxo Methyl butyrate, the yield is 92%;
[0033]
Embodiment 2
[0035] Synthesis of methyl 4-benzyloxy-2-((dimethylamino)methylene)-3-acetoacetate
[0036] Put 40mol of 4-(benzyloxy)-3-oxobutanoic acid methyl ester obtained in Example 1 and 120mol N,N-dimethylformamide dimethyl acetal, 40mol sodium methylate and 400ml toluene into the reaction flask Next, put the reaction bottle into an oil bath, heat to 100°C, and reflux for 5 hours. After the reaction is completed, cool to room temperature, add saturated sodium chloride solution and 3% hydrochloric acid to the reaction bottle, shake for 10 minutes, and divide layer, the organic layer was poured out, and the solvent was removed by a rotary evaporator to obtain a crude product, which was then separated by column chromatography, and the eluent was petroleum ether:methanol=5:1, thereby obtaining 4-benzyl Oxy-2-((dimethylamino)methylene)-3-acetoacetic acid methyl ester, the yield is 95%;
[0037]
Embodiment 3
[0039] Synthesis of dimethyl 4-oxo-3-benzyloxy-4H-pyran-2,5-dicarboxylate
[0040] 30mol of 4-benzyloxy-2-((dimethylamino)methylene)-3-acetoacetate methyl ester obtained in Example 2 and 45mol dimethyl oxalate, 15mol sodium tert-butoxide and 300ml toluene were put into reaction bottle, then put the reaction bottle into an oil bath, heat to 105°C, and reflux for 6h. After the reaction is completed, cool to room temperature, filter, and remove the toluene solvent by rotary evaporation, and then use column chromatography to separate the crude product. The eluent is n-hexane:ethyl acetate=9:4, thus obtaining dimethyl 4-oxo-3-benzyloxy-4H-pyran-2,5-dicarboxylate with a yield of 90%;
[0041]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com