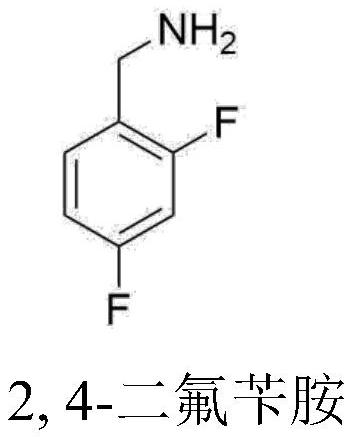
Preparation method of 2,4-difluorobenzylamine
A technology of difluorobenzylamine and difluorobenzonitrile, which is applied in the field of biocatalysis, can solve the problems of low production yield and environmental pollution, and achieve the effect of safe raw materials and environmentally friendly production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 nitrile reductase
[0038] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0039] TB liquid medium composition: peptone 10g / L, yeast powder 18g / L, glycerin 4mL / L, KH 2 PO 4 2.31g / L, K2 HPO 4 2H 2 O 16.43g / L, dissolved in deionized water to constant volume, sterilized at 121°C for 20min, ready for use.
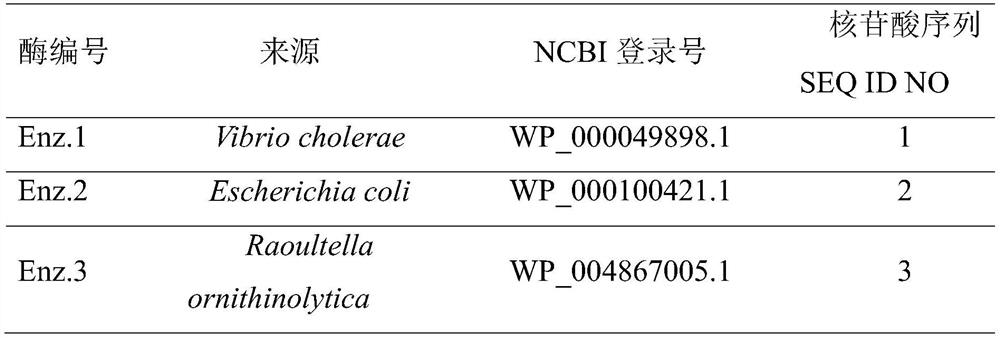
[0040] See Table 1 below for the amino acid and nucleotide sequence information of nitrile reductase. Suzhou Jinweizhi Biotechnology Co., Ltd. (No. 211 Pubin Road, Yanchuang Park, Jiangbei New District, Nanjing, Jiangsu Province) synthesized the nitrile reductase (Enz.1~Enz.3) gene (SEQ IDNO:1-3) from the whole gene, digested with enzymes The sites NdeI and HindIII were connected into the vector pET28a. The synthetic nitrile reductase gene is transformed into host E. coli BL21 (DE3) competent cells to ...
Embodiment 2
[0051] The preparation of embodiment 2 glucose dehydrogenase
[0052] According to the glucose dehydrogenase (GDH) gene sequence derived from Bacillus subtilis 168 (NCBI accession number NP_388275.1), Suzhou Jinweizhi Biotechnology Co., Ltd. (Pubin, Jiangbei New District, Nanjing, Jiangsu Province) Road No. 211) whole gene synthesis of glucose dehydrogenase (GDH) gene, restriction site NdeI, HindIII, connected into vector pET28a. The synthetic GDH gene was transformed into host E.coli BL21 (DE3) competent cells to obtain engineering strains containing the GDH gene.
[0053] After the engineered bacteria containing the GDH gene were activated by streaking on the plate, a single colony was picked and inoculated into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37 °C for 4 h. Transfer to 150mL fresh TB liquid medium containing 50μg / mL kanamycin at 1v / v% inoculum amount, shake culture at 37°C until OD600 reaches about 0.8, add IPTG to its fina...
Embodiment 3
[0058] Example 3 Nitrile reductase catalyzes 2,4-difluorobenzonitrile to prepare 2,4-difluorobenzylamine
[0059]
[0060] Add 50mL of pure water, 6.5g of glucose, NADP+0.5mg to the reaction system, add 20mL of isopropanol, 5g of 2,4-difluorobenzonitrile, stir and dissolve in a water bath at 30°C, and dissolve with 20% aqueous sodium carbonate (mass volume ratio) to fine-tune the pH to 7.2-7.5, and finally add 20 mL of the liquid nitrile reductase obtained in Example 1 (2.5 g of bacteria slime plus buffer homogeneously obtained) and 10 mL of GDH prepared in Example 2. The reaction was continued in a water bath at 30°C, pH 7.4, 200 rpm. During the reaction, the conversion rate of the substrate was detected by HPLC. The results are shown in Table 3 below.
[0061]
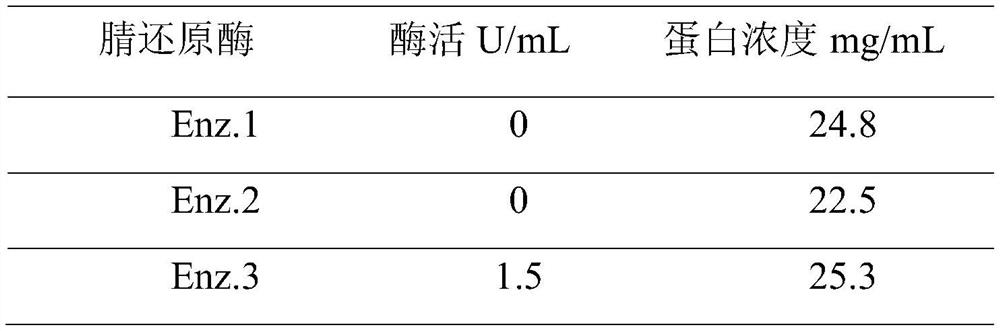
[0062] The 20 hour conversion of nitrile reductase Enz.03 as shown in the table above was 70%. In this example, the reaction system or reaction conditions are adjusted, for example, when the reaction temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com