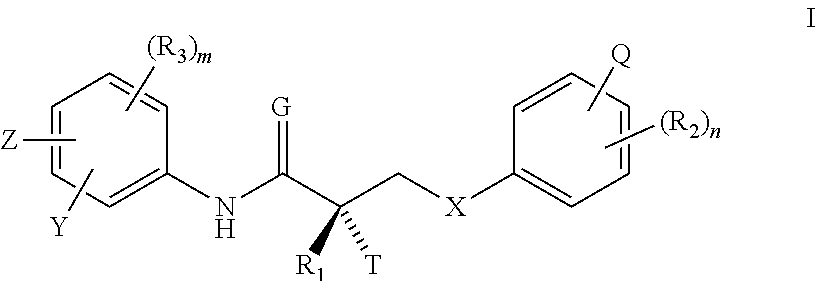
Patents
Literature
61 results about "Pertuzumab" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pertuzumab is used with other medications to treat certain types of breast cancer that has spread to other parts of the body in patients who have not received treatment or chemotherapy. It is also used with other medications to treat early stage breast cancer in patients who will be having surgery to remove the cancer. The types of cancers pertuzumab is used to treat are tumors that produce more than the normal amount of a certain substance called HER2 protein.
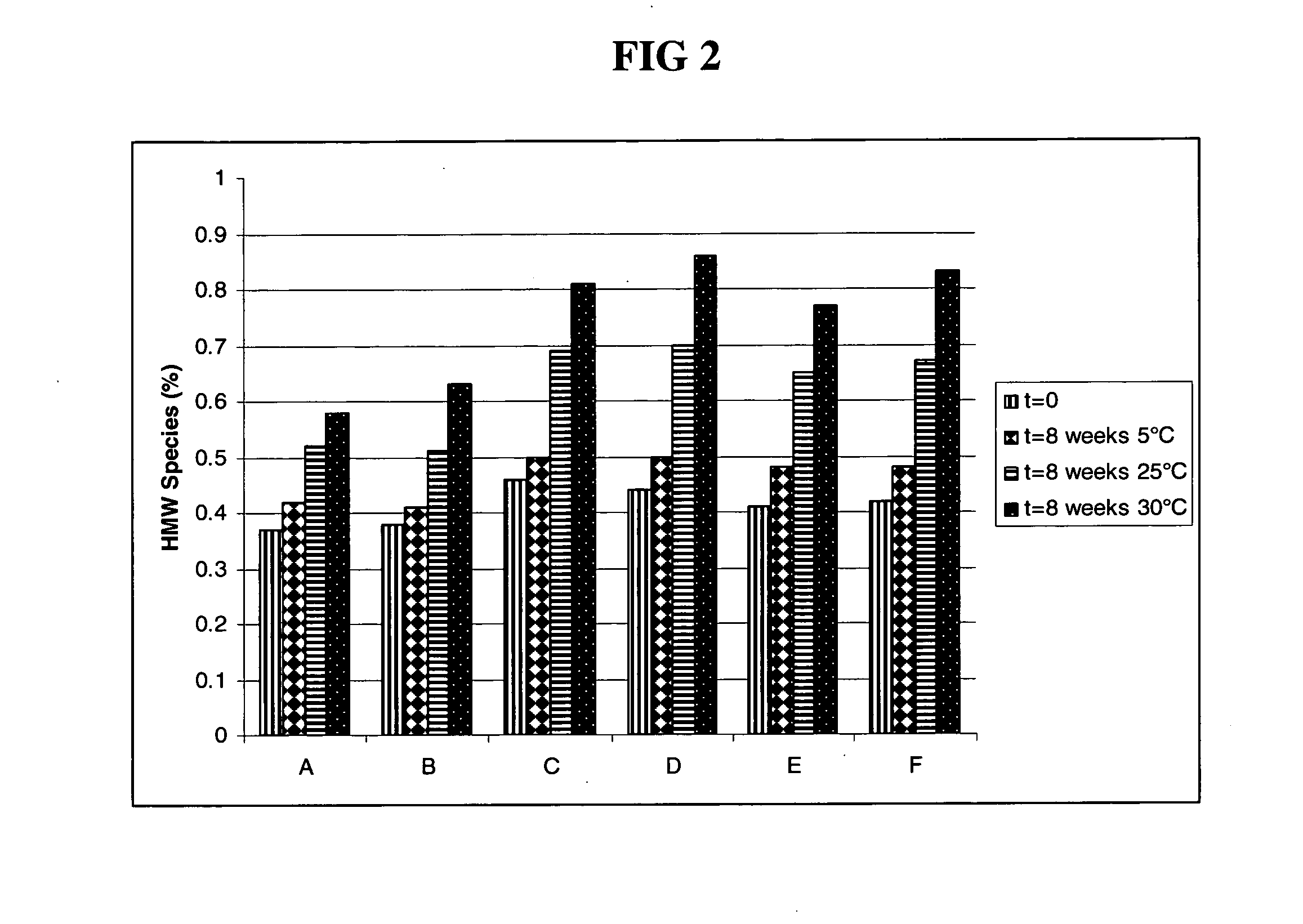
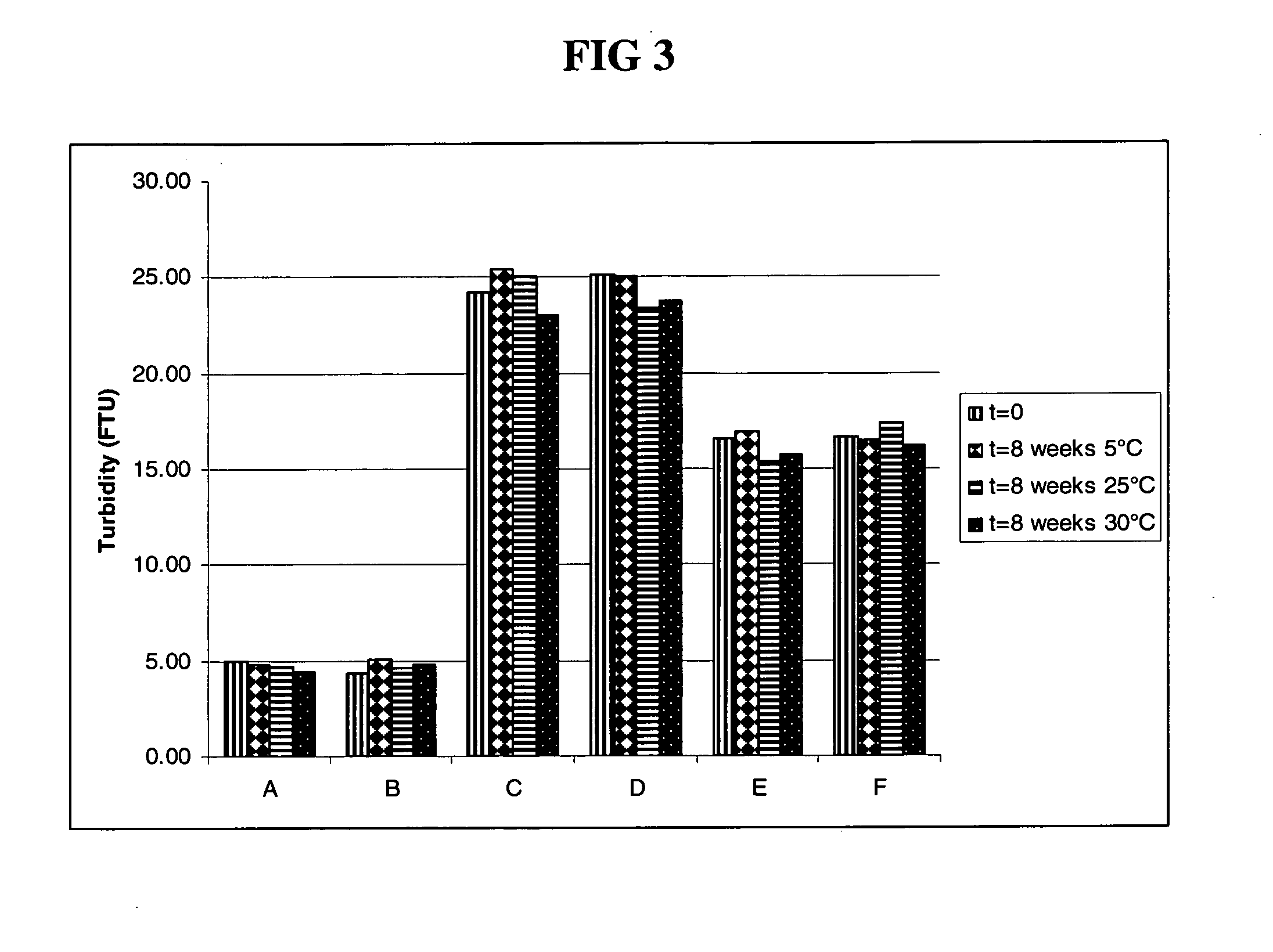
Antibody formulations
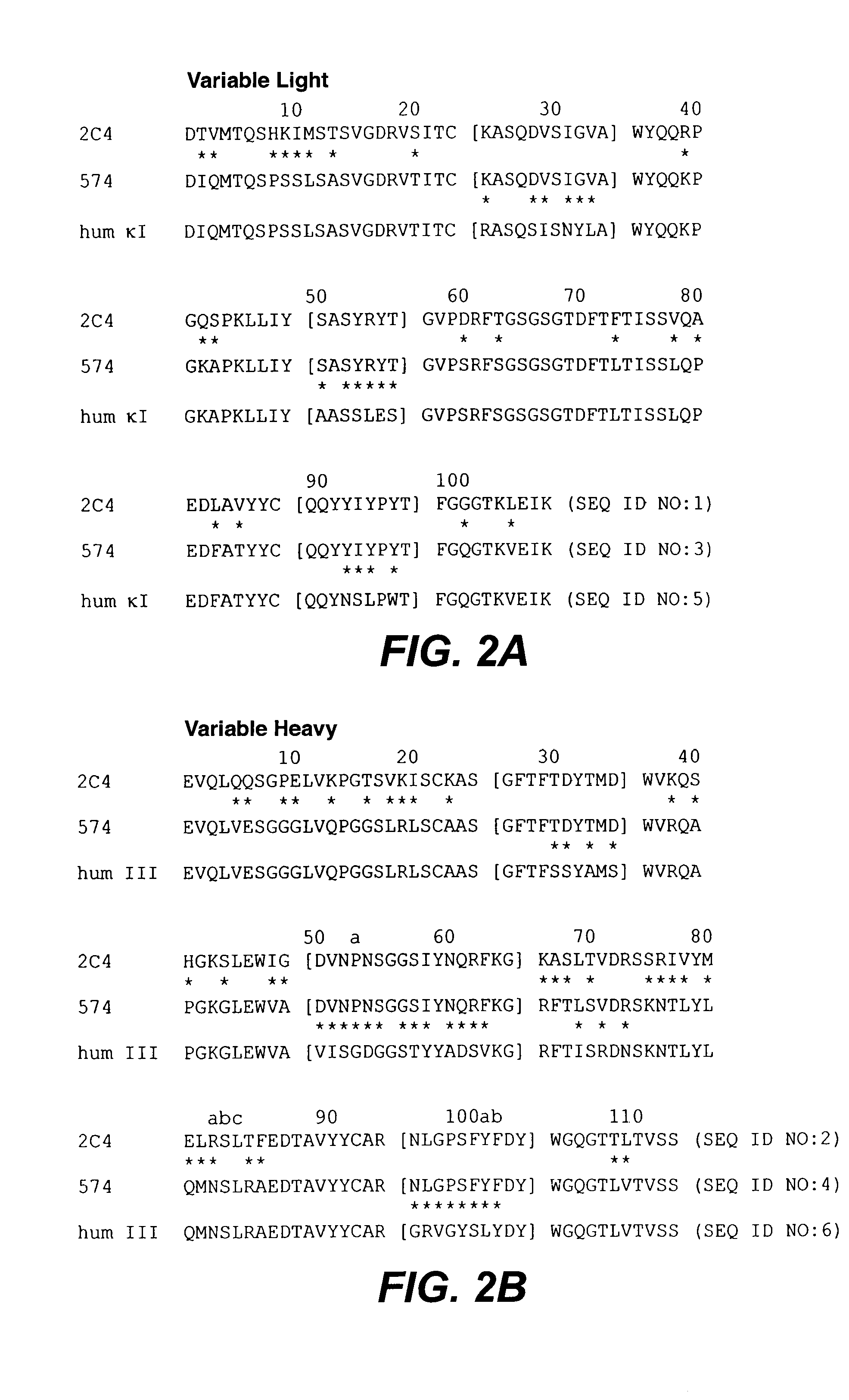
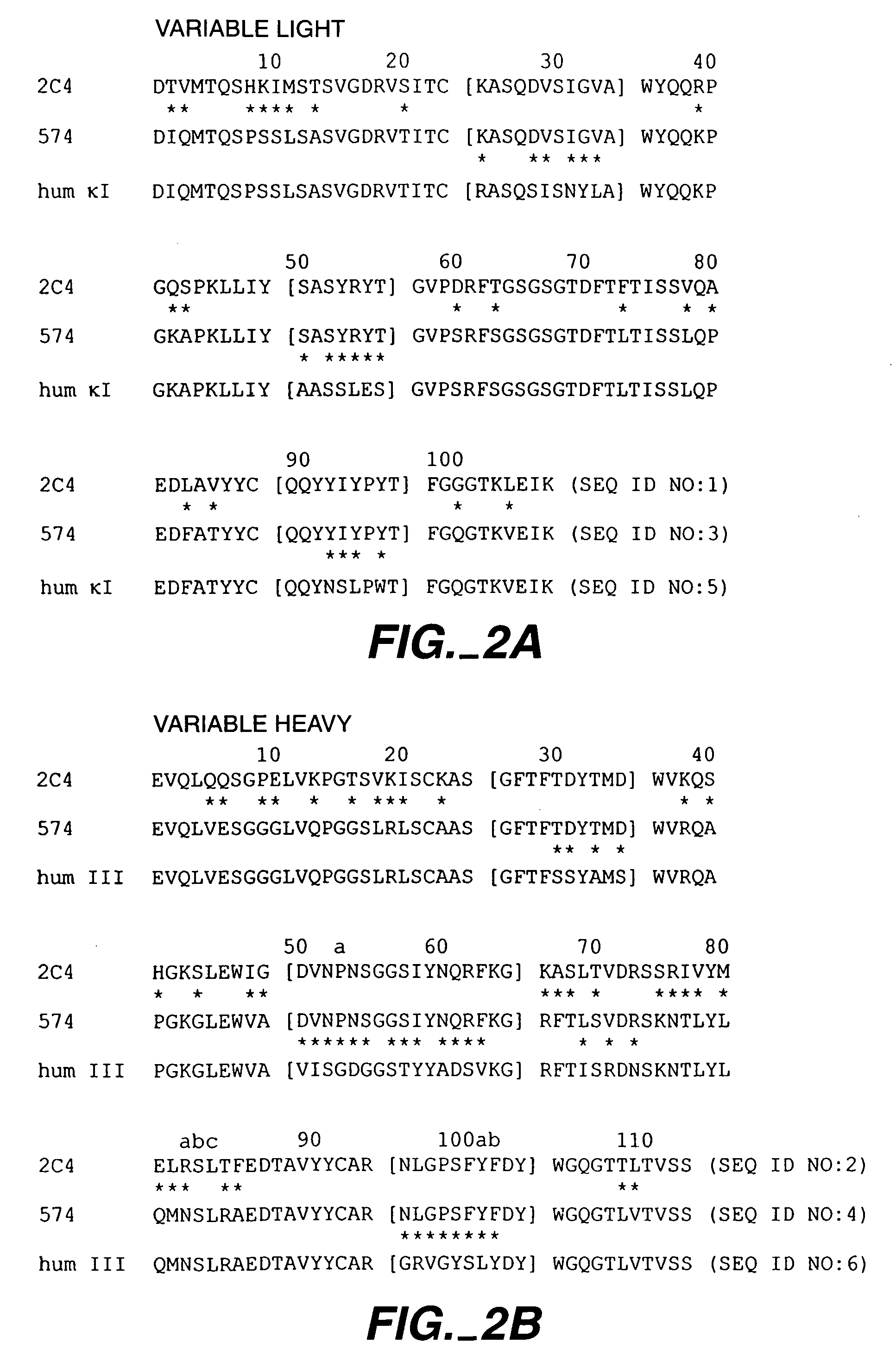
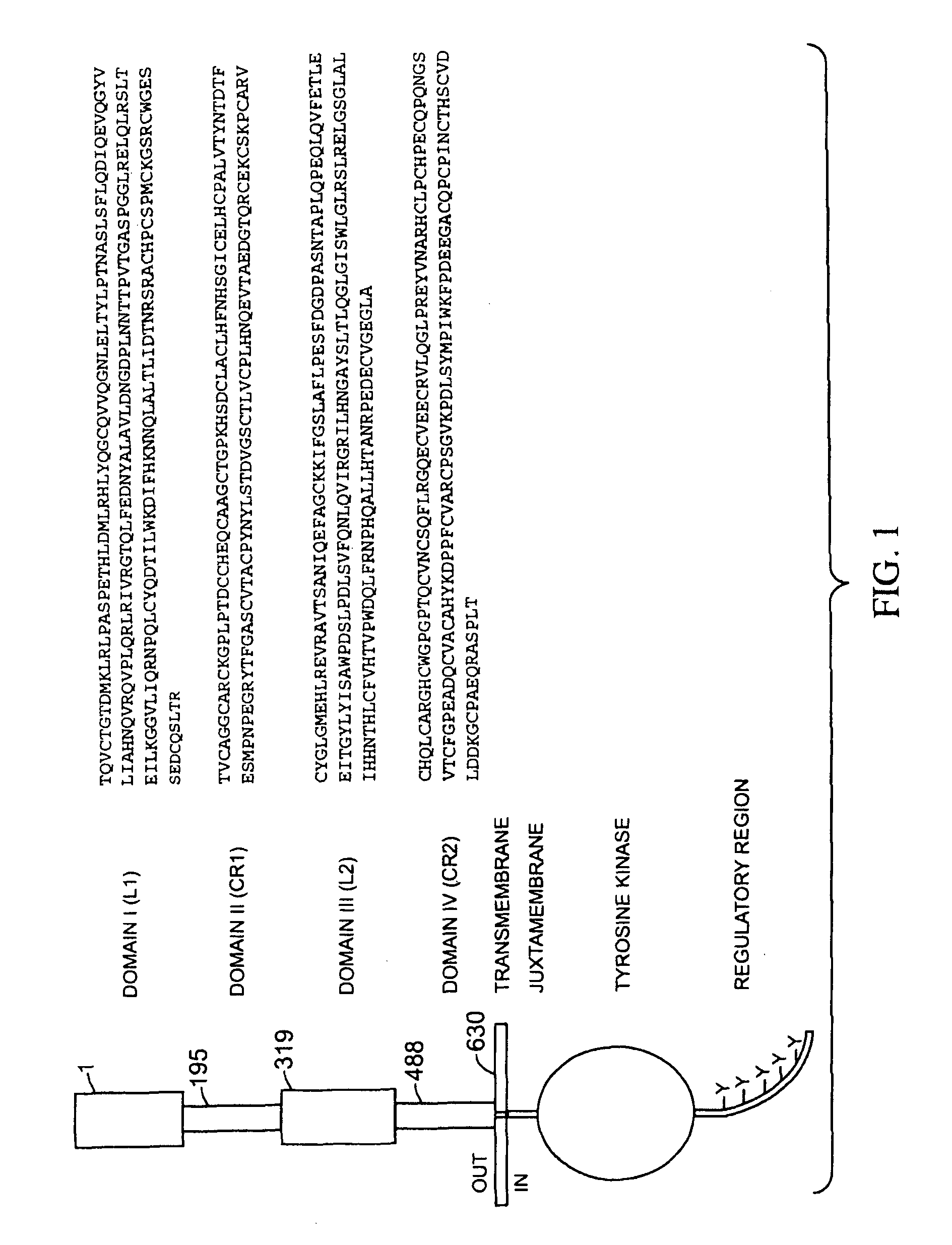
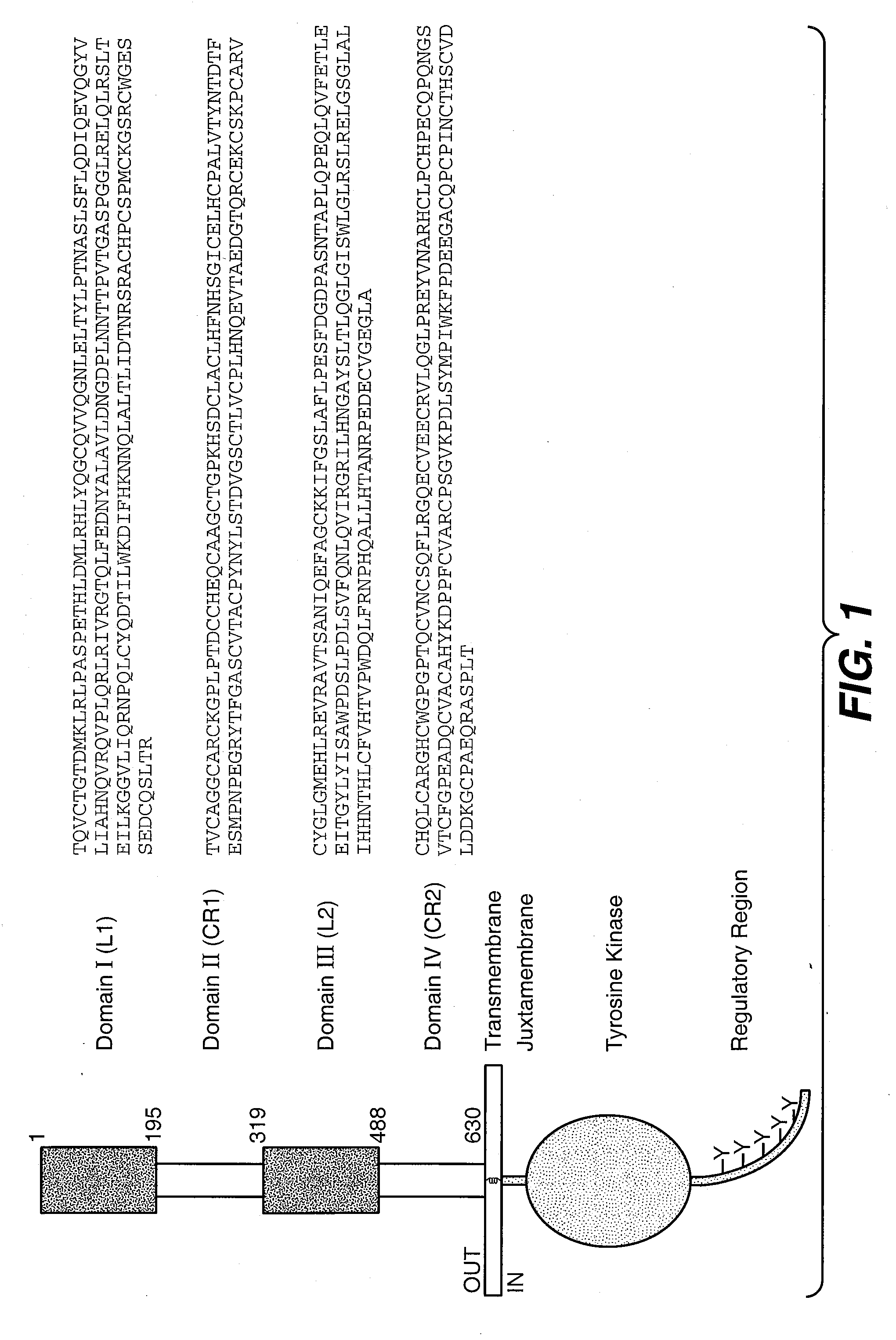
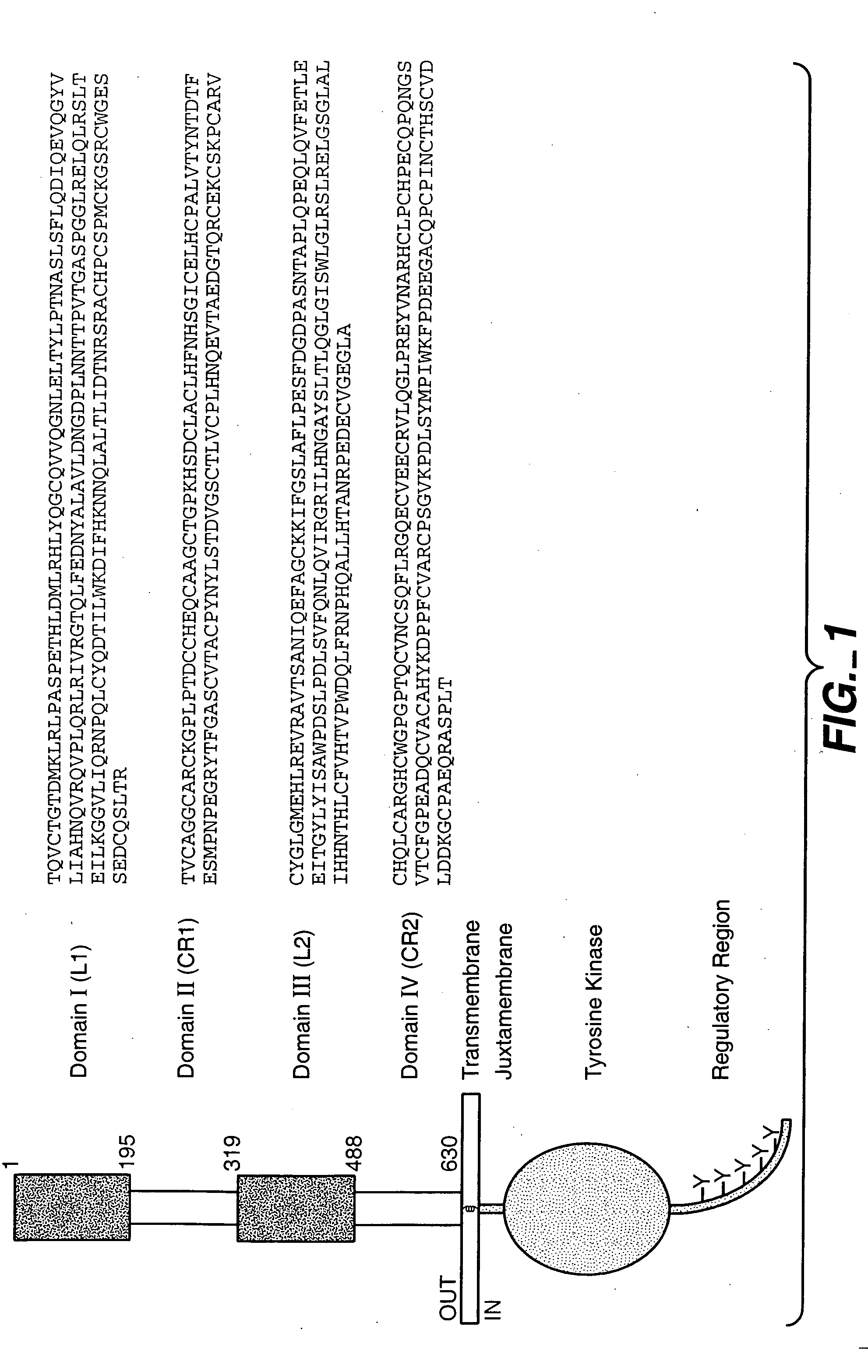
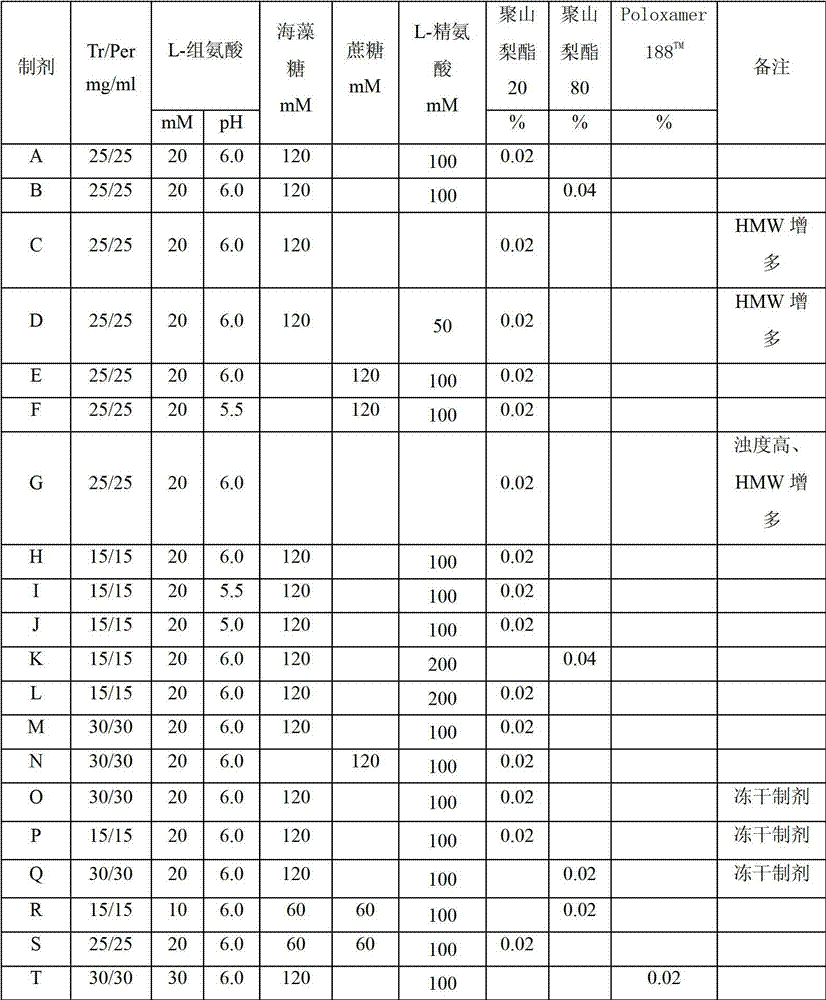
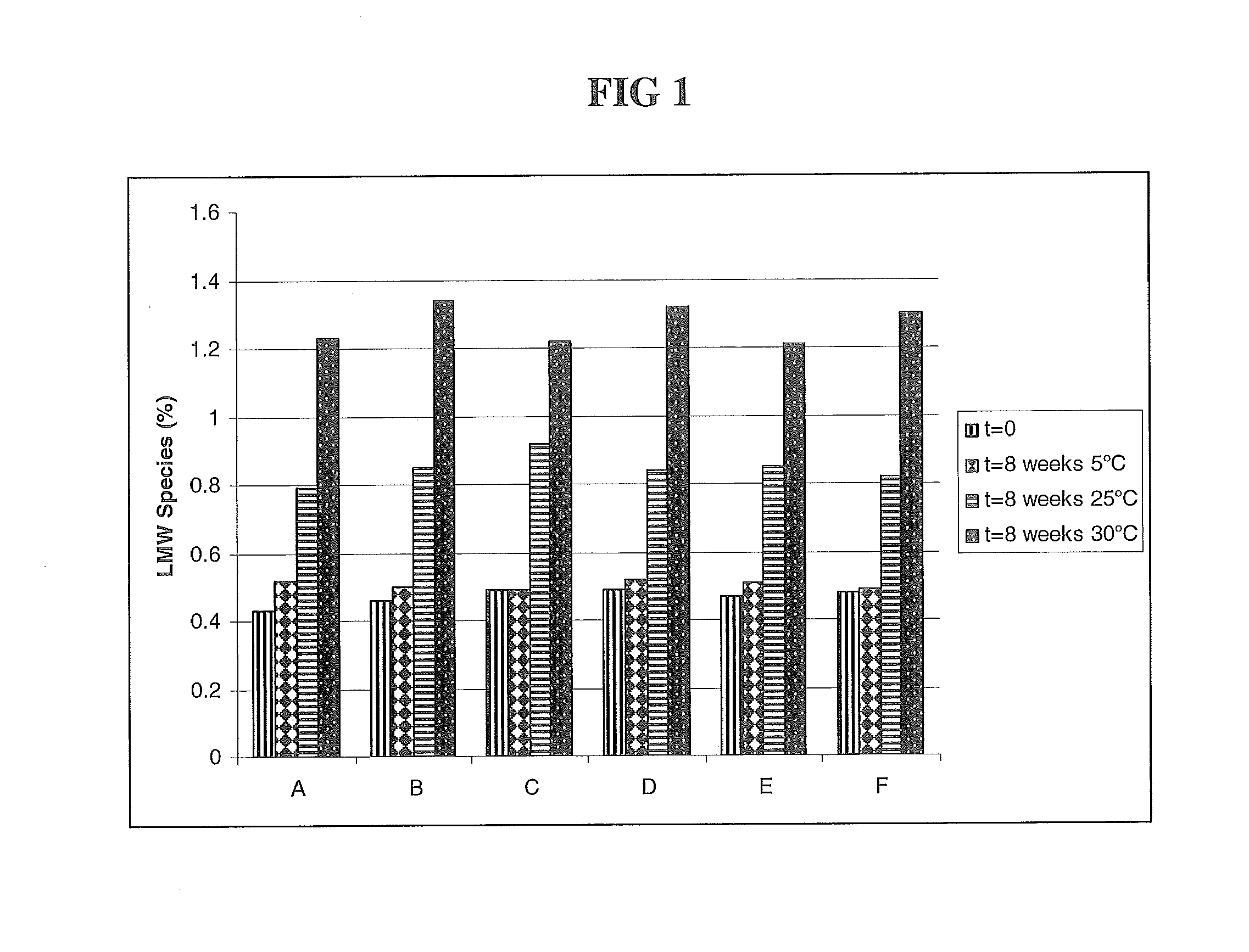
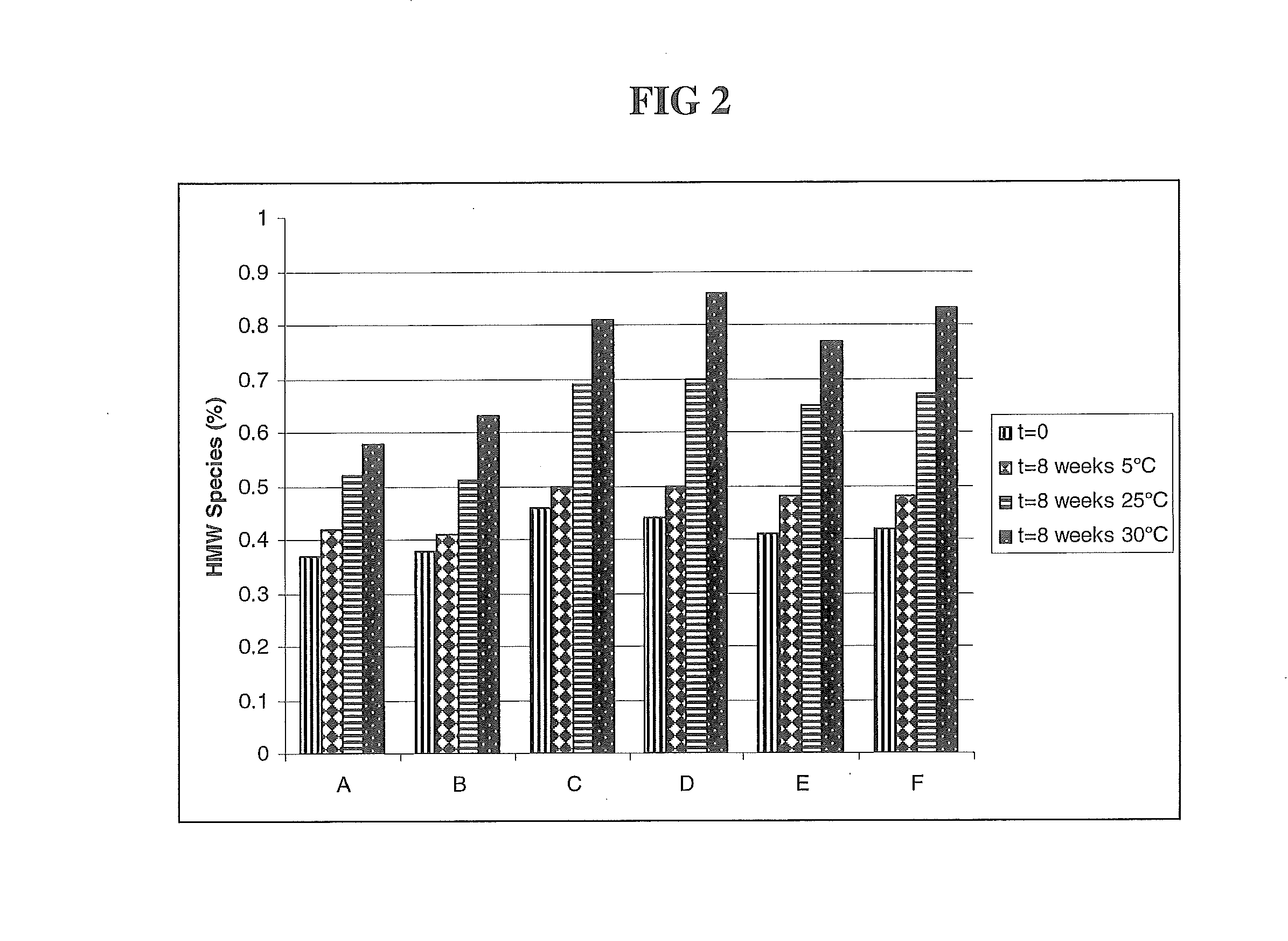
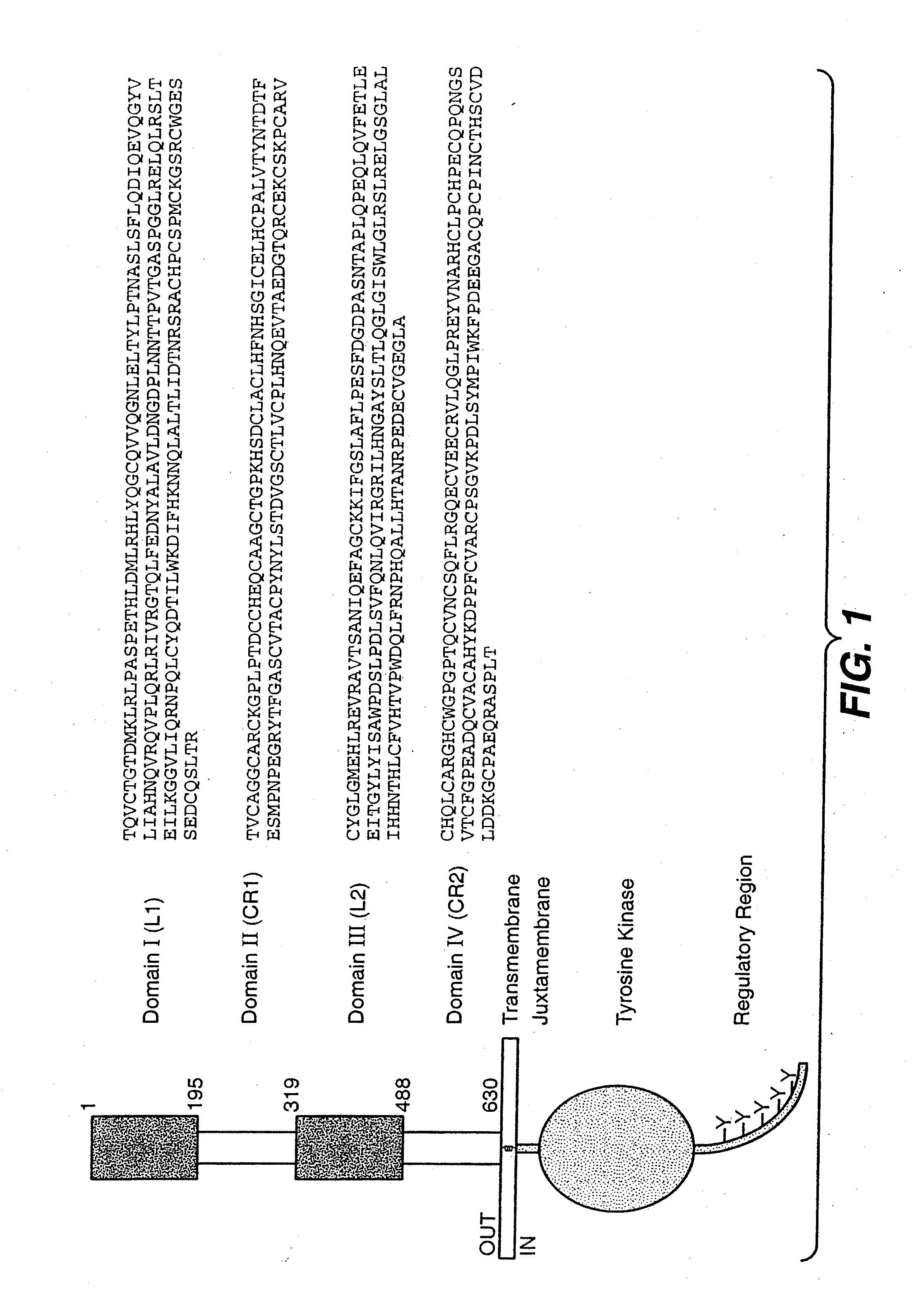
The present application describes antibody formulations, including monoclonal antibodies formulated in histidine-acetate buffer, as well as a formulation comprising an antibody that binds to domain II of HER2 (for example, Pertuzumab), and a formulation comprising an antibody that binds to DR5 (for example, Apomab).
Owner:GENENTECH INC
Antibody formulations
The present application describes antibody formulations, including monoclonal antibodies formulated in histidine-acetate buffer, as well as a formulation comprising an antibody that binds to domain II of HER2 (for example, Pertuzumab), and a formulation comprising an antibody that binds to DR5 (for example, Apomab).
Owner:GENENTECH INC
Fixed dosing of HER antibodies
Owner:GENENTECH INC
Subcutaneous anti-HER2 antibody formulations and uses thereof
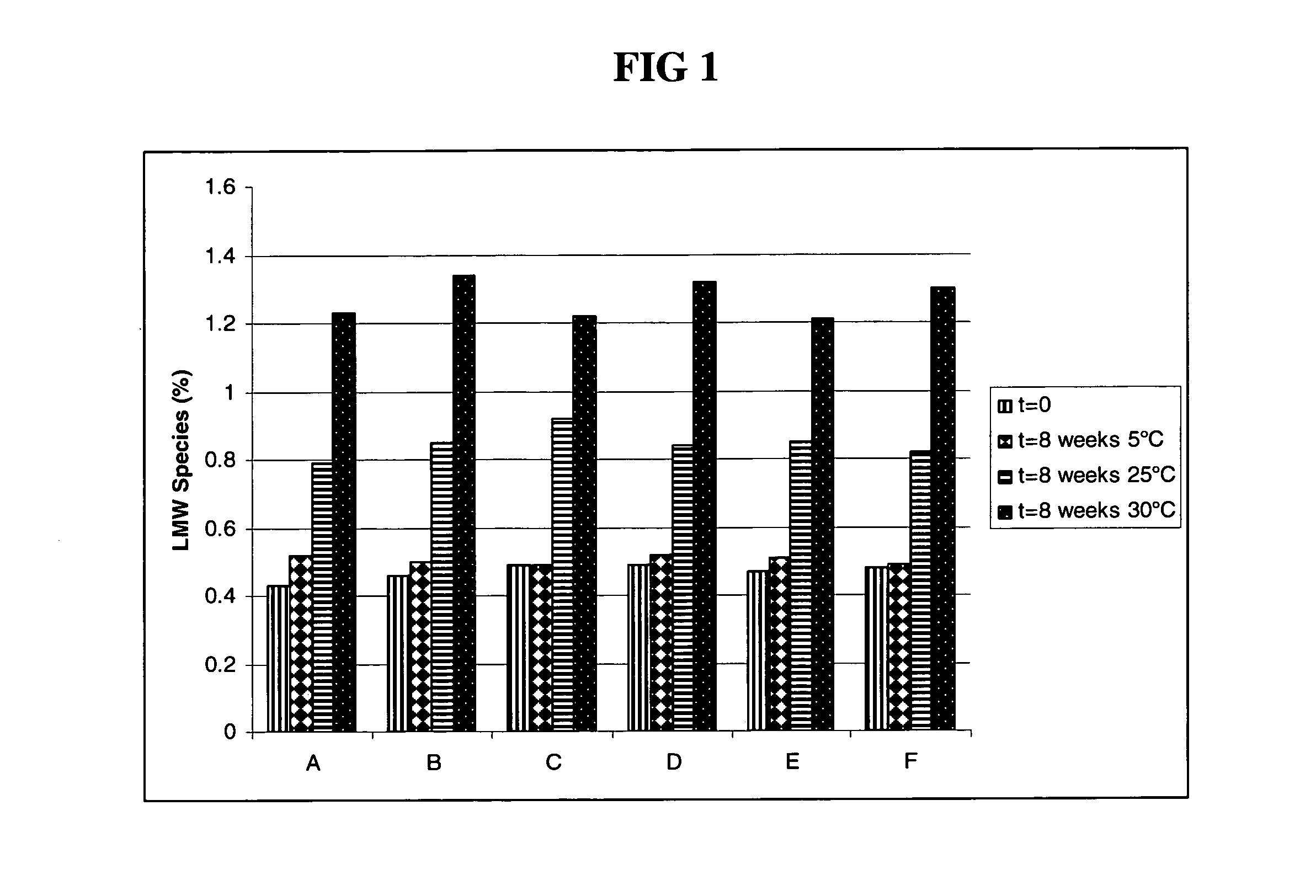
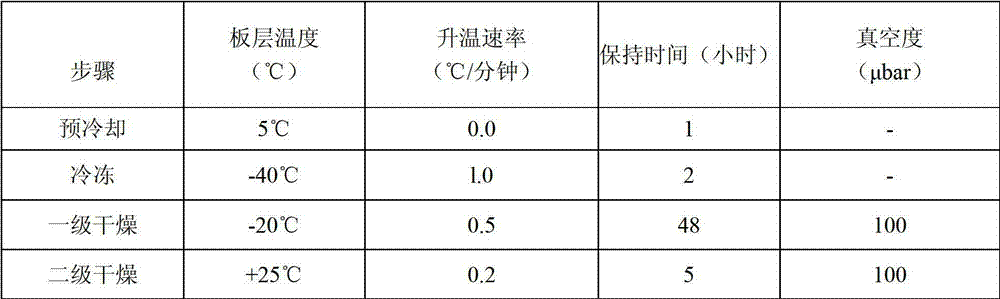
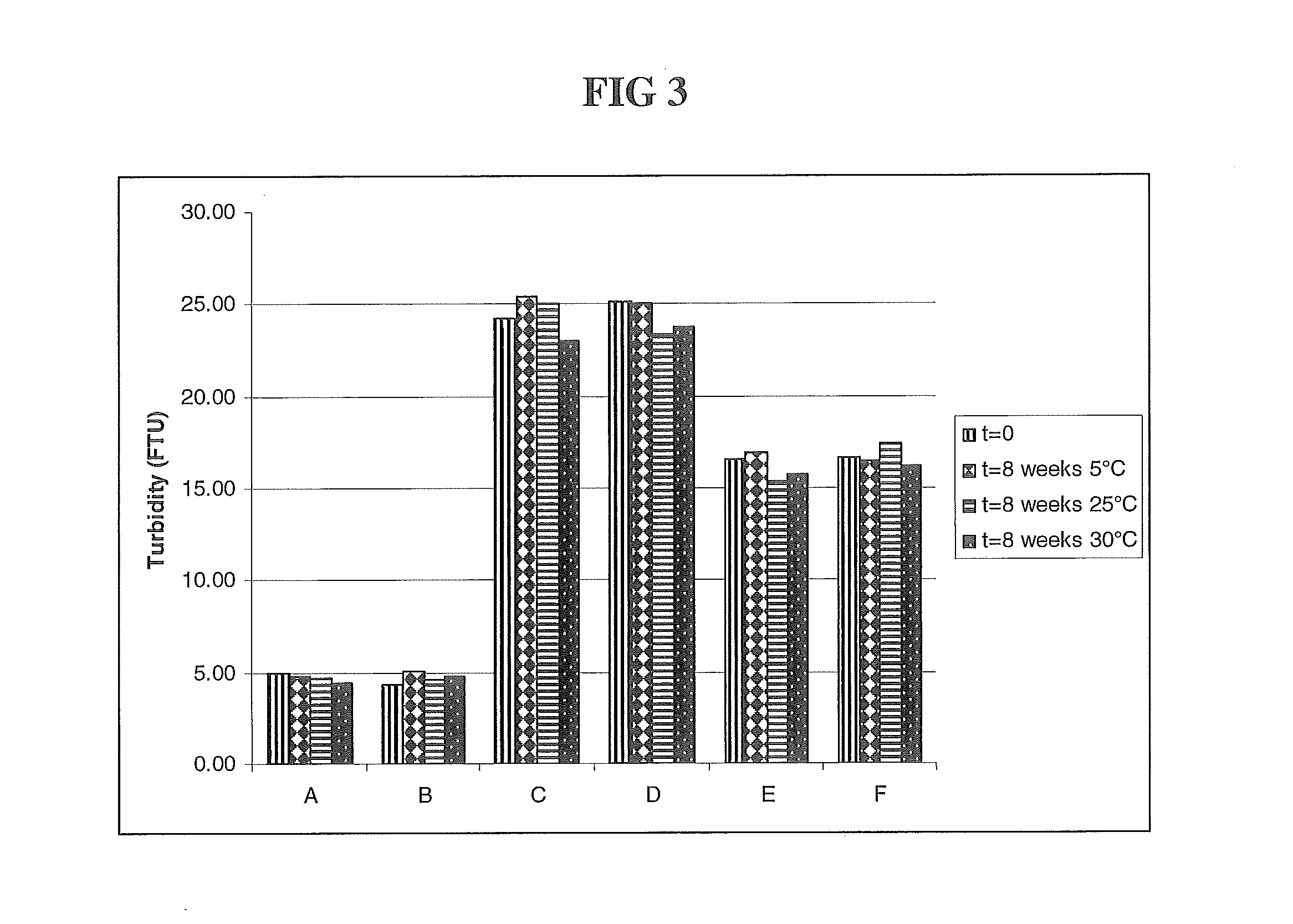
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Predicting response to a HER inhibitor
InactiveUS7981418B2Improve responseOrganic active ingredientsData processing applicationsObstetricsSelection criterion
The present application describes the use of low HER3 as a selection criterion for treating patients with a HER inhibitor, such as pertuzumab.It also describes the use of high HER2:HER3 ratio as a selection criterion for treating cancer patients, such as ovarian cancer patients, with a HER inhibitor, such as pertuzumab.In addition, the application describes the use of high HER3 as a selection criterion for treating cancer patients with a chemotherapeutic agent, for instance gemcitabine.
Owner:F HOFFMANN LA ROCHE & CO AG
Treatment of metastatic breast cancer
InactiveUS20090317387A1Improve survivalOrganic active ingredientsAntibody ingredientsBiologic therapiesDocetaxel-PNP
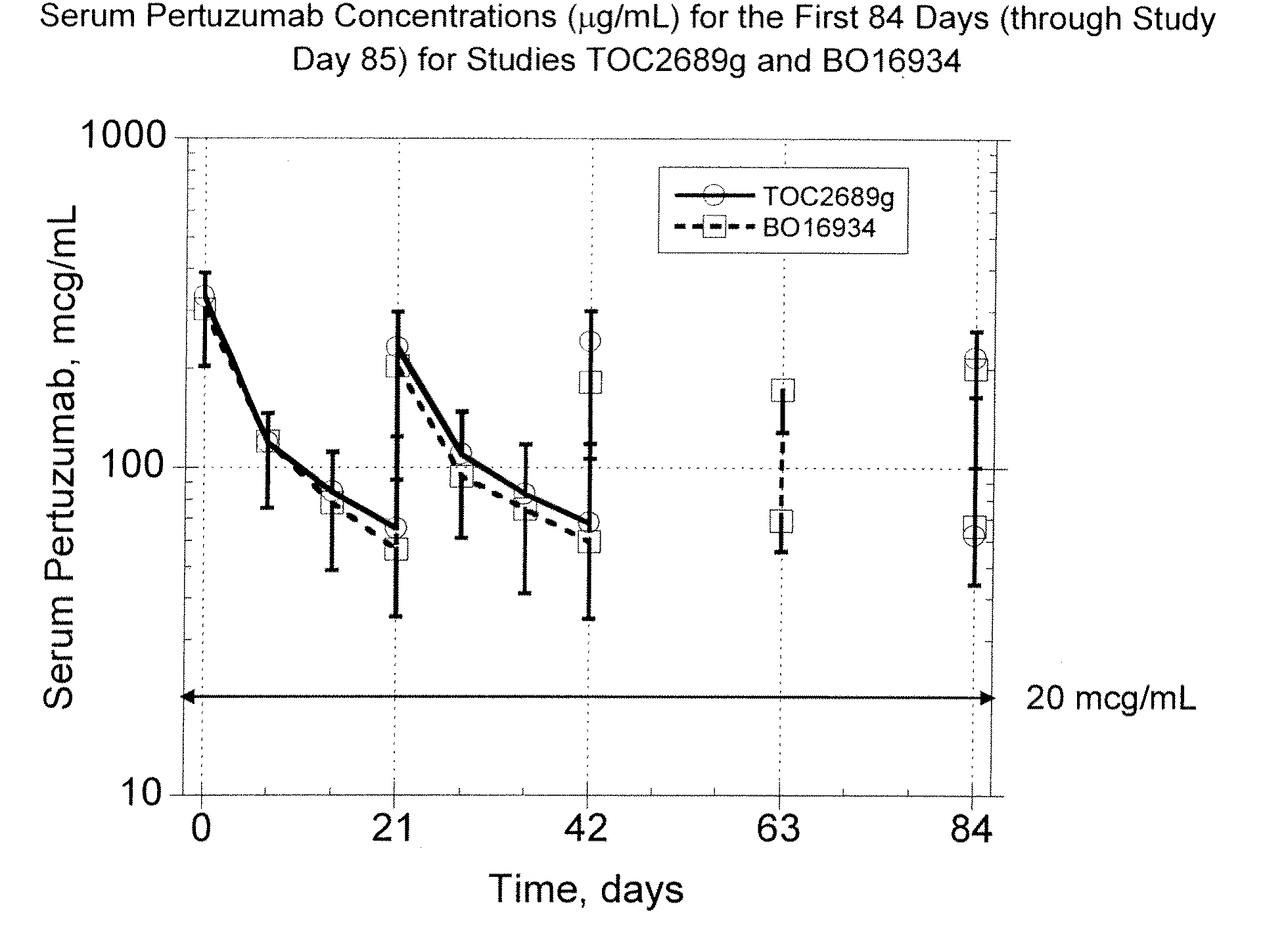
The present invention concerns treatment of previously untreated HER2-positive metastatic breast cancer with a combination of a growth inhibitory HER2 antibody, a HER2 dimerization inhibitor antibody and a taxane. In particular, the invention concerns the treatment of HER2-positive metastatic breast cancer in patients who did not receive prior chemotherapy or biologic therapy with a HER2 antibody binding essentially to epitope 2C4, a HER2 antibody binding essentially to epitope 4D5, and a taxane. The invention further comprises extending survival of such patients by the combination therapy of the present invention. In a preferred embodiment, the treatment involves administration of trastuzumab, pertuzumab and docetaxel.
Owner:GENENTECH INC
Extending time to disease progression or survival in cancer patients
InactiveUS20060188509A1Extension of timeExtends TTPOrganic active ingredientsDigestive systemBiological activationSurgery
The present application describes extending time to disease progression or survival in a cancer patient, where the patient's cancer displays HER activation, by treating the patient with a HER dimerization inhibitor, such as pertuzumab.
Owner:GENENTECH INC
Extending time to disease progression or survival in cancer patients
InactiveUS20090155259A1Extension of timeExtends TTPOrganic active ingredientsDigestive systemBiological activationSurgery
The present application describes extending time to disease progression or survival in a cancer patient, where the patient's cancer displays HER activation, by treating the patient with a HER dimerization inhibitor, such as pertuzumab.
Owner:GENENTECH INC
Fixed dosing of HER antibodies
ActiveUS20060165702A1Effective treatmentOrganic active ingredientsPeptide/protein ingredientsAntibodyPertuzumab
Owner:GENENTECH INC
Selecting patients for therapy with a her inhibitor
InactiveUS20080317753A1Effective treatmentEffective amountPeptide/protein ingredientsMicrobiological testing/measurementPhosphorylationHER Inhibitors
A method for selecting patients for therapy with a HER inhibitor, such as pertuzumab, based on gene expression analysis is described. A method for assessing HER phosphorylation or activation in a biological sample via gene expression analysis is also described.
Owner:GENENTECH INC
Subcutaneous anti-HER2 antibody formulations and uses thereof
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Method of treating estrogen receptor (ER) -positive breast cancers with selective androgen receptor modulator (SARMS)
This invention relates to the treatment of androgen receptor-positive breast cancer in a subject, for example a female subject. Accordingly, this invention provides methods of: a) treating a subject suffering from breast cancer; b) treating a subject suffering from metastatic breast cancer; c) treating a subject suffering from refractory breast cancer; d) treating a subject suffering from AR-positive breast cancer; e) treating a subject suffering from AR-positive refractory breast cancer; f) treating a subject suffering from AR-positive metastatic breast cancer; g) treating a subject suffering from AR-positive and ER-positive breast cancer; h) treating a subject suffering from triple negative breast cancer; i) treating a subject suffering from advanced breast cancer; j) treating a subject suffering from breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; k) treating, preventing, suppressing or inhibiting metastasis in a subject suffering from breast cancer; l) prolonging survival of a subject with breast cancer, and / or m) prolonging the progression-free survival of a subject with breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound, comprising administering to the subject a therapeutically effective amount of a SARM compound of this invention.
Owner:UNIV OF TENNESSEE RES FOUND
Method of treating androgen receptor (AR) -positive breast cancers with selective androgen receptor modulator (SARMS)
ActiveUS20140350102A1Treating and preventing and suppressing and inhibiting metastasisProlonged progression-free survivalBiocideOrganic chemistryToremifeneLymphatic Spread
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer advanced breast cancer; breast cancer that has failed SERM (tamoxifen, toremifene), aromatase inhibitor, trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, exemestane (Aromasin), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
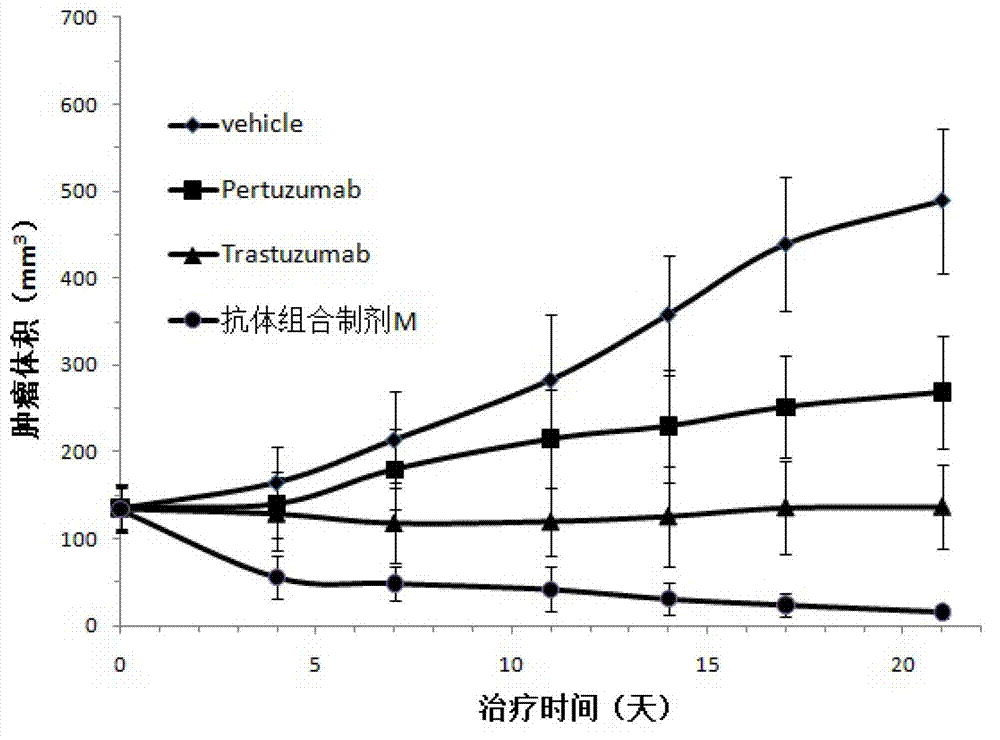
Antibody composition preparation and application thereof
ActiveCN102961745AAvoid formingAvoid gatheringAntibody ingredientsAntineoplastic agentsAnti her2Pertuzumab
Relating to the technical field of pharmaceutical preparations, the invention provides an antibody composition preparation composed of Trastuzumab and Pertuzumab. The antibody composition preparation has better tumor treatment activity. Compared with the independent treatment of Trastuzumab and Pertuzumab, the composition preparation shows a synergistic effect of the two antibodies, and can reach a tumor control rate of 92.43%. And the preparation provided in the invention has better stability, thus being in favor of long-term preservation of the antibody composition preparation. The antibody composition preparation provided in the invention is applicable to preparation of drugs treating diseases or symptoms suited to anti-HER2 antibody therapy. During application, the antibody composition preparation disclosed in the invention is used with a chemotherapeutic agent jointly or sequentially.
Owner:SUZHOU KANGJU BIOTECHNOLOGY CO LTD +1
Subcutaneous anti-HER2 Antibody Formulations and Uses Thereof
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Treatment of metastatic breast cancer
InactiveUS20140044704A1Improve survivalOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDocetaxel-PNPDocetaxel
The present invention concerns treatment of previously untreated HER2-positive metastatic breast cancer with a combination of a growth inhibitory HER2 antibody, a HER2 dimerization inhibitor antibody and a taxane. In particular, the invention concerns the treatment of HER2-positive metastatic breast cancer in patients who did not receive prior chemotherapy or biologic therapy with a HER2 antibody binding essentially to epitope 2C4, a HER2 antibody binding essentially to epitope 4D5, and a taxane. The invention further comprises extending survival of such patients by the combination therapy of the present invention. In a preferred embodiment, the treatment involves administration of trastuzumab, pertuzumab and docetaxel.
Owner:GENENTECH INC
Methods of treating early breast cancer with trastuzumab-mcc-dm1 and pertuzumab
InactiveUS20170035907A1Improve responseExtension of timeOrganic active ingredientsAntibody ingredientsGynecologyEarly breast cancer
Methods of treating patients having HER2-positive, operable, locally advanced or inflammatory breast cancer with the antibody-drug conjugate Trastuzumab-MCC-DM1 and Pertuzumab are provided.
Owner:GENENTECH INC
Methods of treating her2-positive cancer
InactiveUS20180251557A1The dosage is easy to controlEnable patient complianceOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHER2 Positive Breast CancerClinical settings
Methods of treating patients having HER2-positive cancer are provided. Certain methods involve treatment of HER2 positive breast cancer using a programmed cell death protein 1 (PD-1) binding antagonist or a programmed death ligand 1 (PD-L1) binding antagonist in combination with trastuzumab and pertuzumab or with trastuzumab emtansine. The treatment regimen may be used in various clinical settings, for example, for treatment in the neoadjuvant or metastatic setting.
Owner:GENENTECH INC
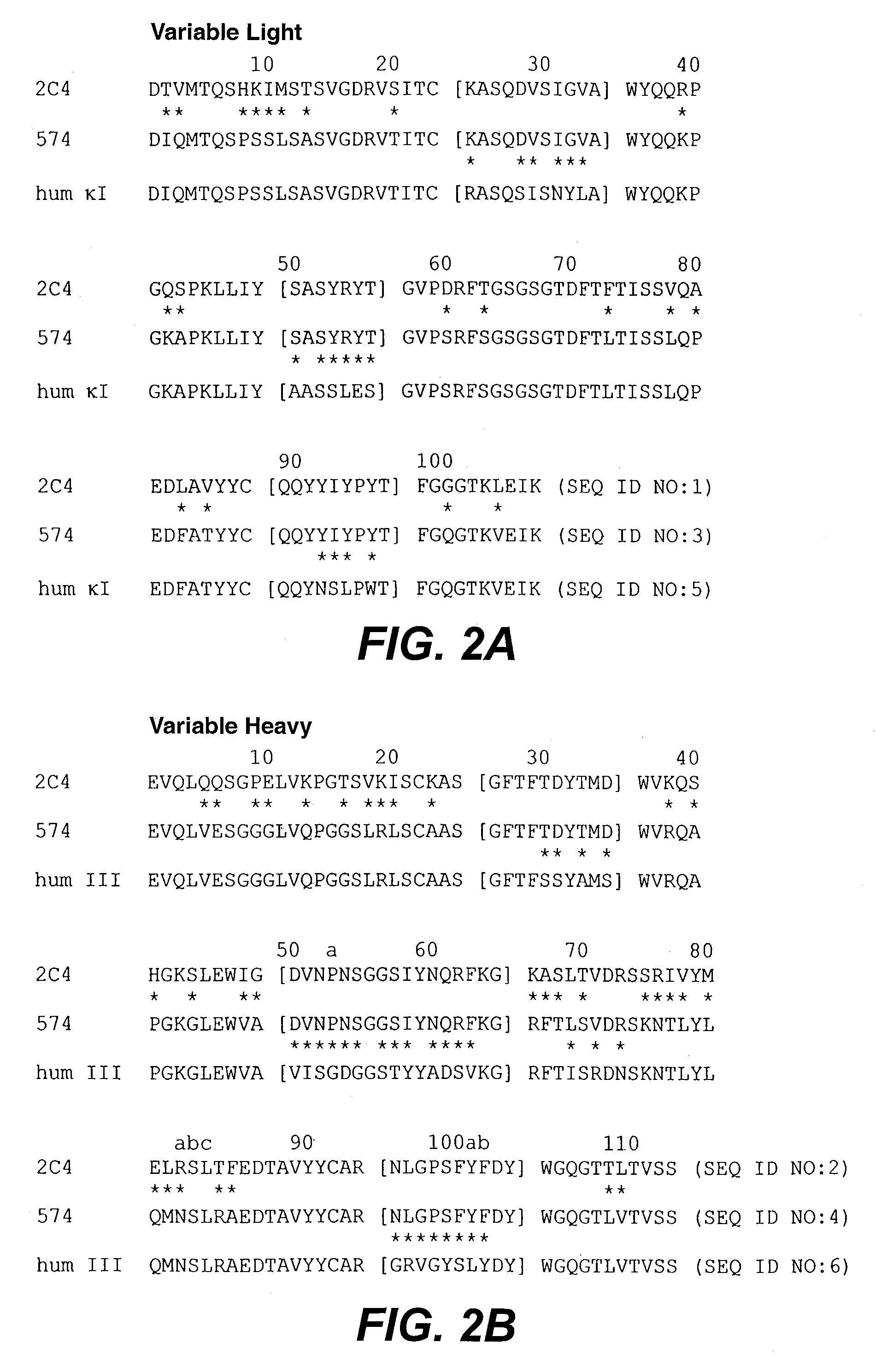
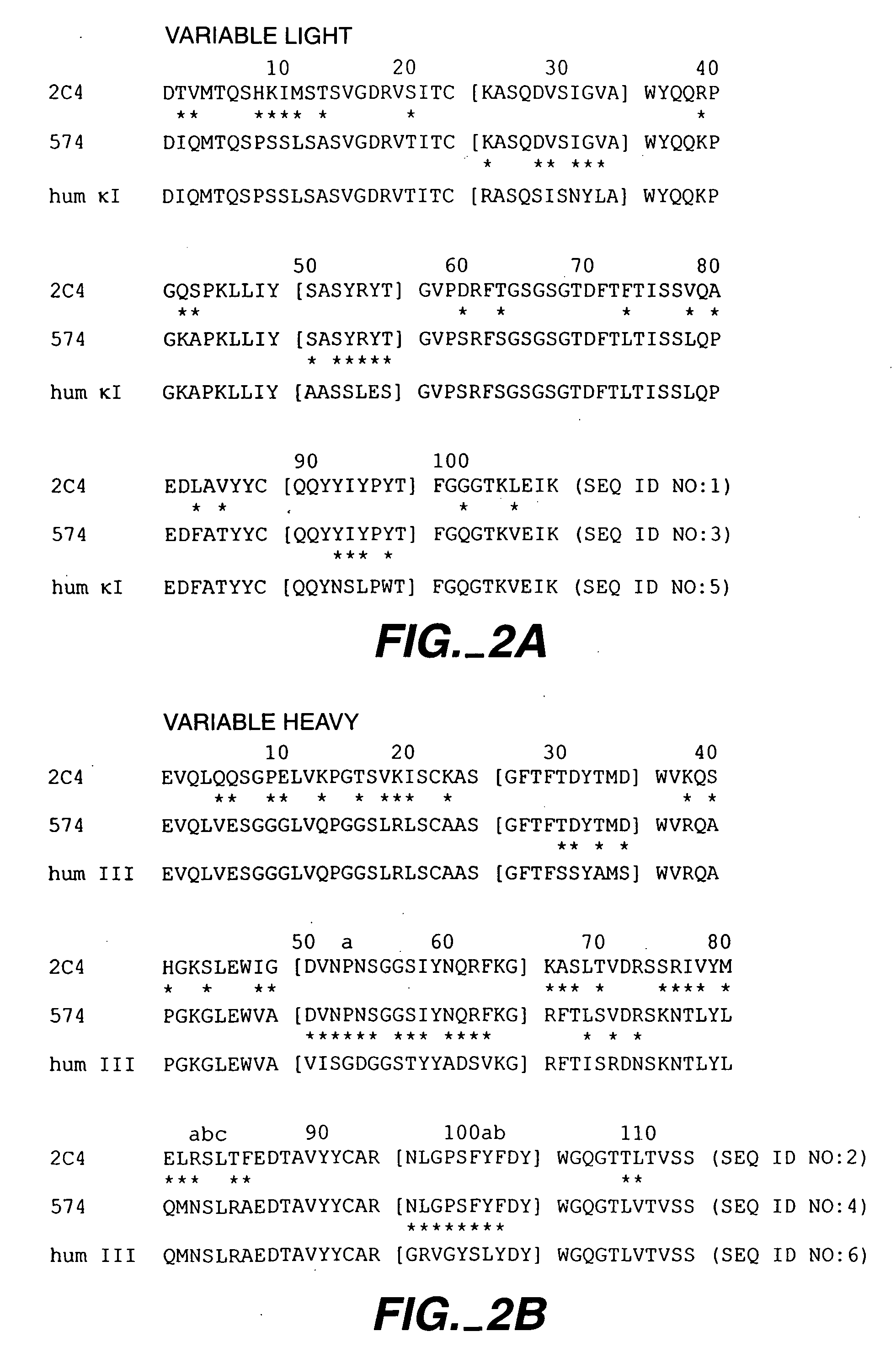
Bispecific Anti-her2 antibody
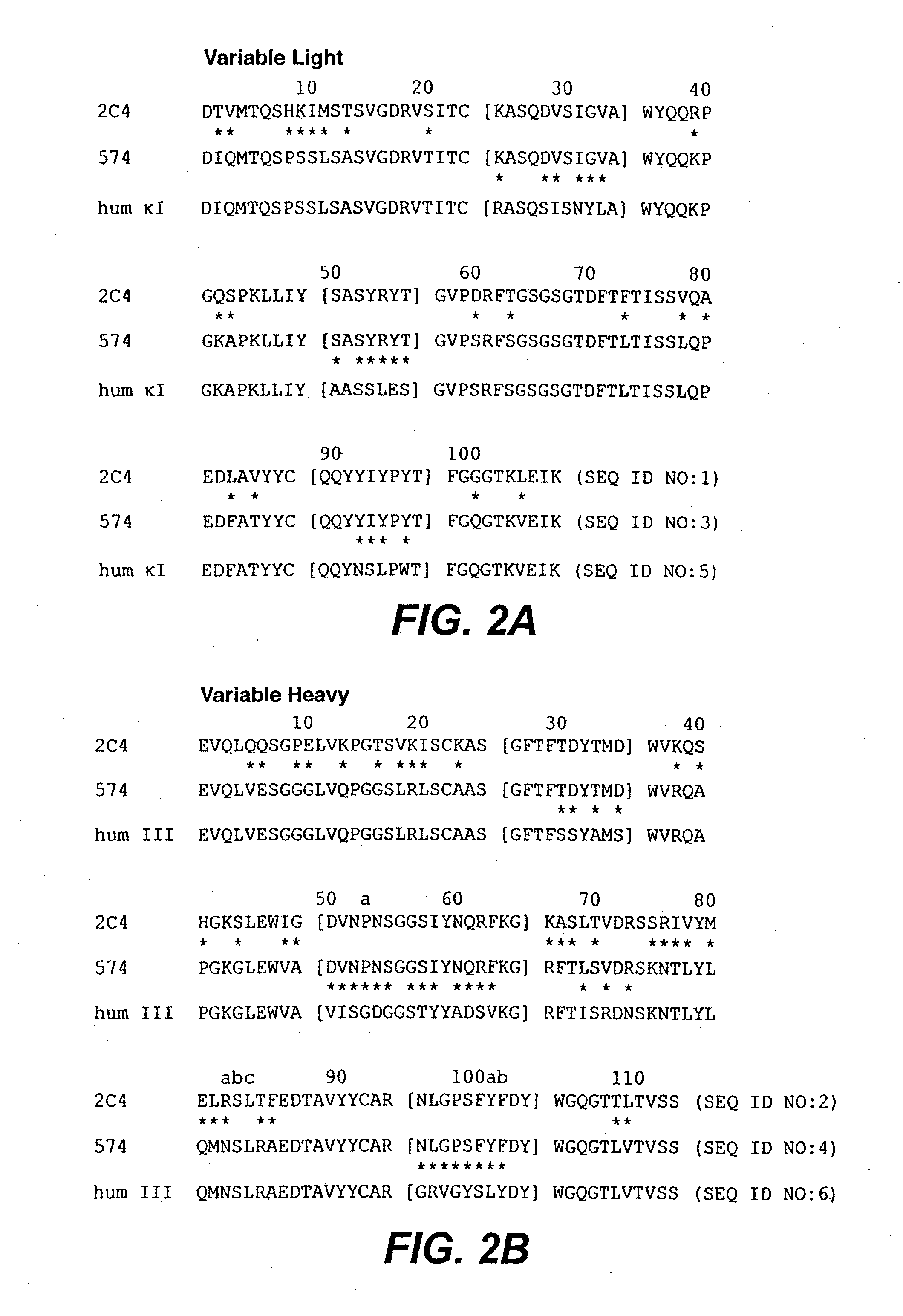
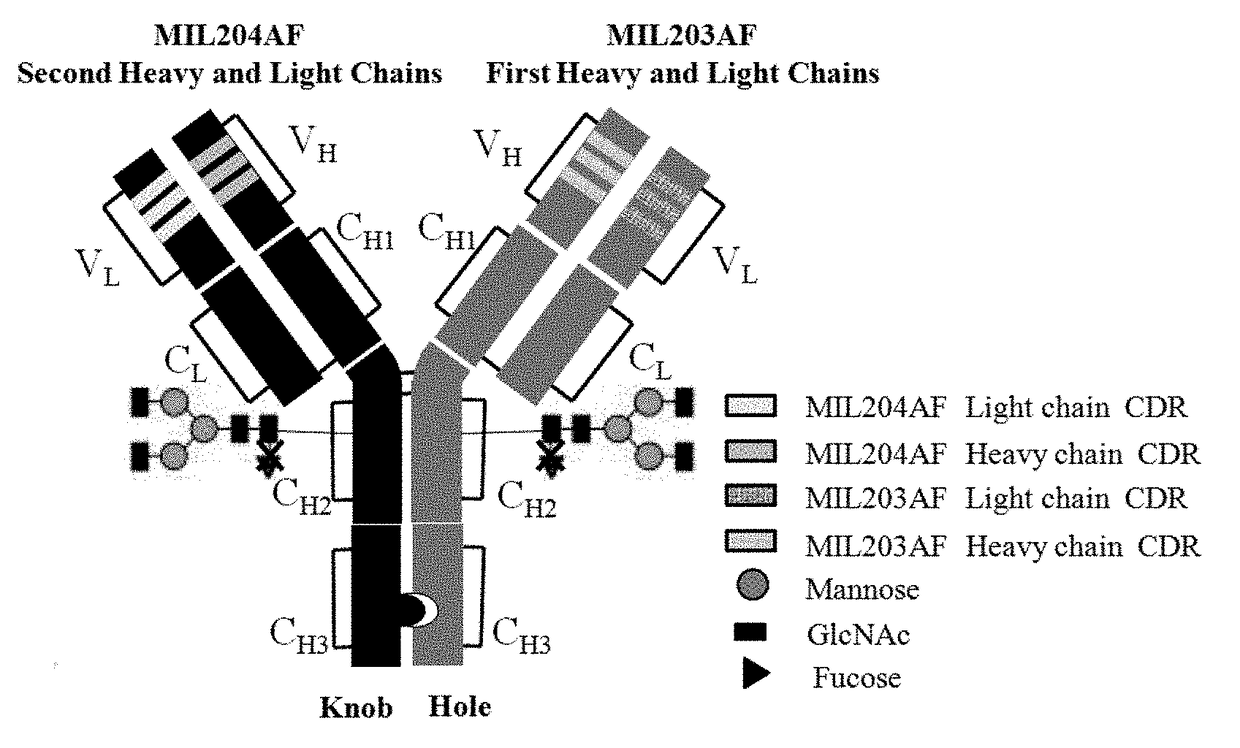
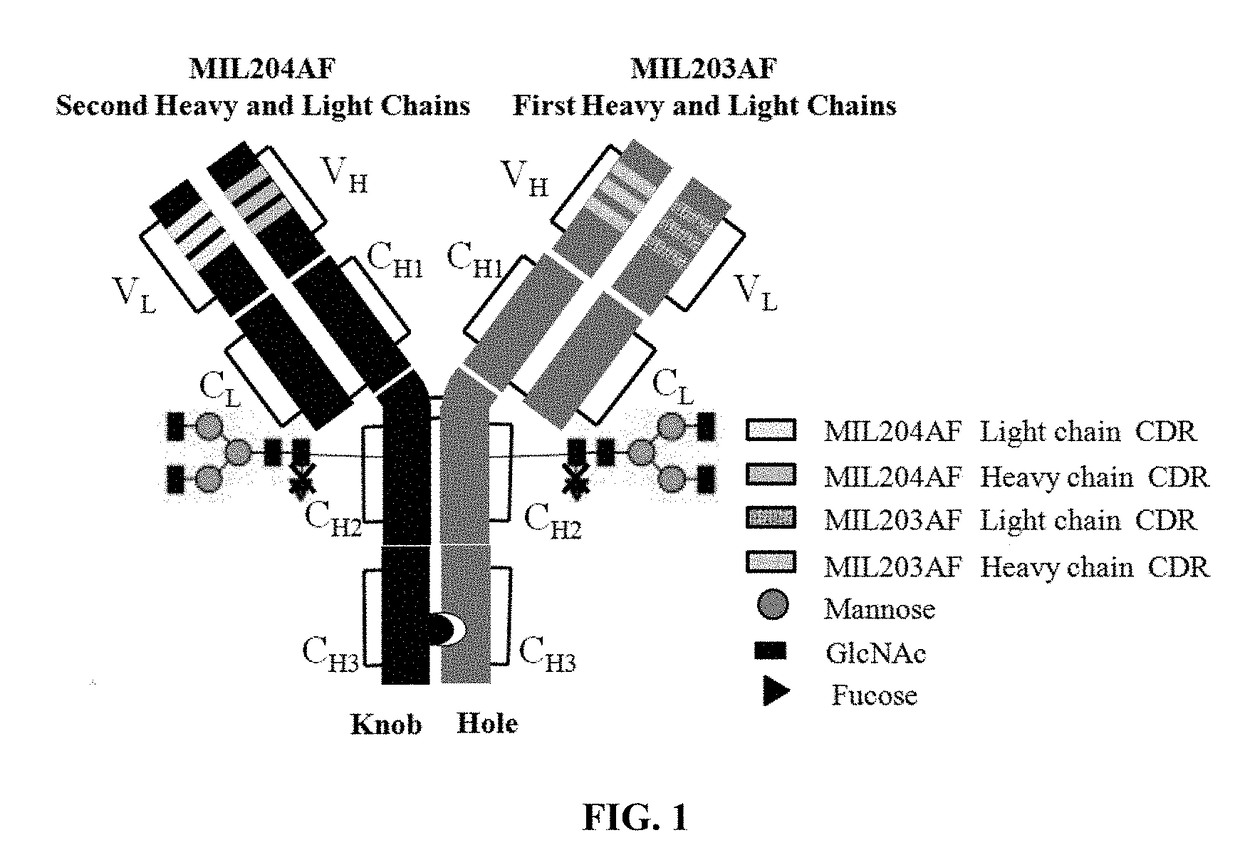
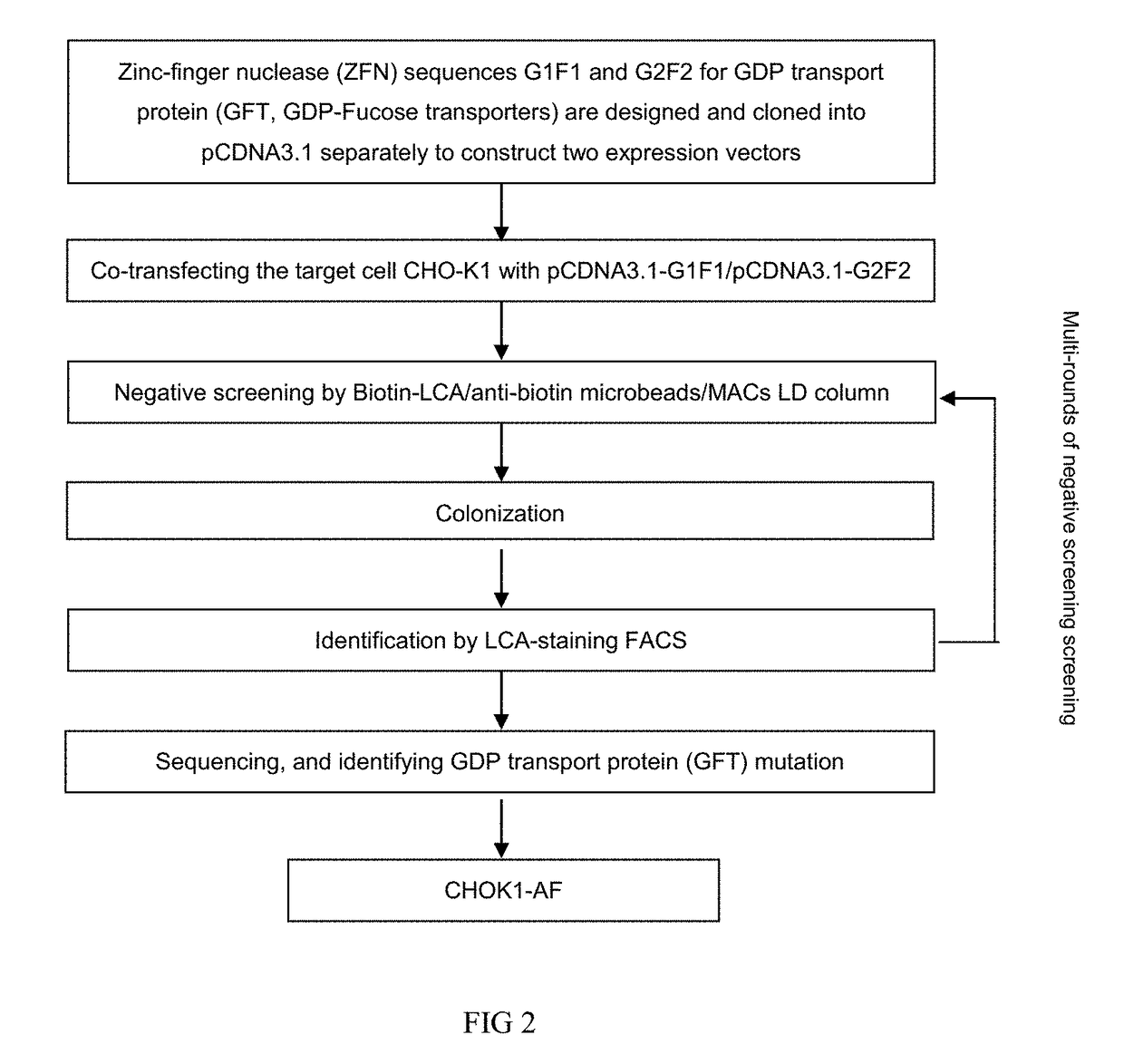
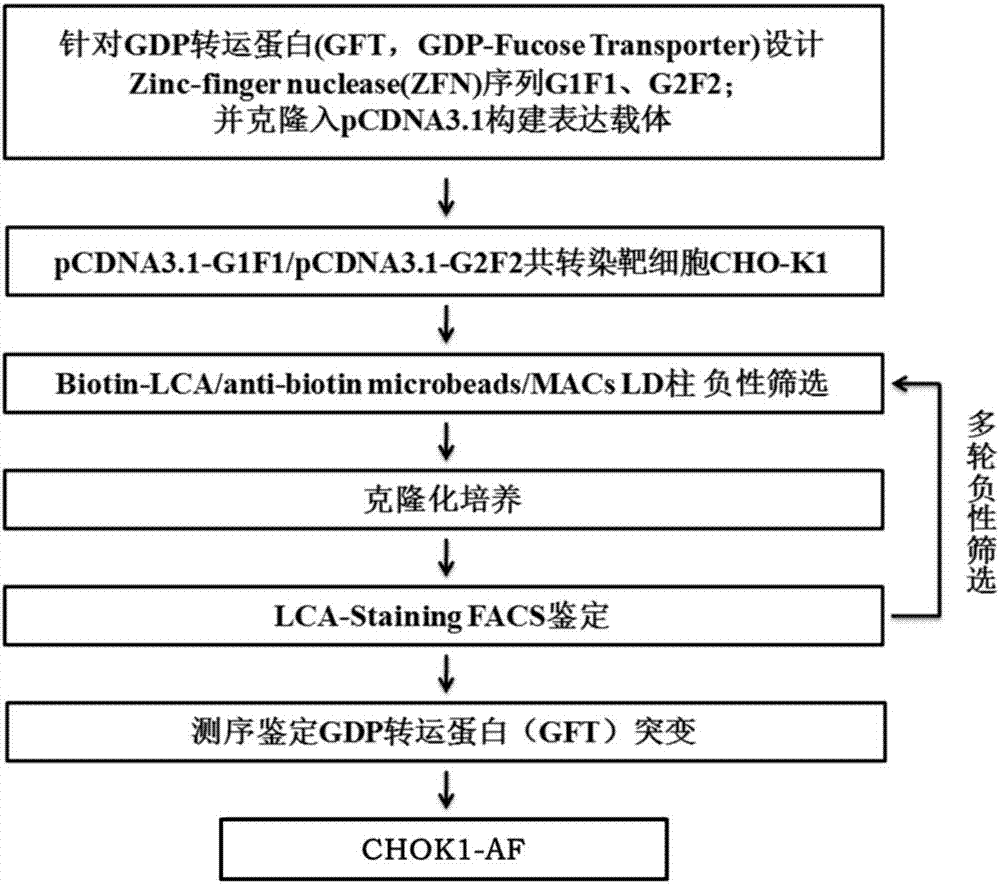
The present invention relates to humanized bispecific anti-HER2 antibodies that comprise one antigen binding site containing variable regions of heavy and light chain of trastuzumab, and another antigen binding site containing variable regions of heavy and light chain of pertuzumab. The bispecific anti-HER2 antibodies is effective for treating cancer, such as breast cancer, gastric cancer, or ovarian cancer. Preferred bispecific anti-HER antibodies of the present invention are afucosylated antibodies. The present invention also relates to Chinese Hamster ovary (CHO) mutant cell line that has a dysfunctional Slc35C1 gene, which is the only dysfunctional gene in the mutant that affects glycan regulation.
Owner:BEIJING MABWORKS BIOTECH
Adjuvant treatment of her2-positive breast cancer
ActiveUS20180250397A1Reduce riskReduce deathOrganic active ingredientsPharmaceutical containersPrimary breast cancerEarly breast cancer
Methods are provided for the adjuvant treatment of operable HER2-positive primary breast cancer in human patients by administration of pertuzumab in addition to chemotherapy and trastuzumab. The methods reduce the risk of recurrence of invasive breast cancer or death for a patient diagnosed with HER2-positive early breast cancer (eBC) compared to administration of trastuzumab and chemotherapy, without pertuzumab.
Owner:F HOFFMANN LA ROCHE & CO AG
Subcutaneous her2 antibody formulations
ActiveUS20180296470A1Good dispersionOrganic active ingredientsPeptide/protein ingredientsCancer therapyPertuzumab
Fixed dose HER2 antibody formulations for subcutaneous administration are provided along with their use in the treatment of cancer. The formulations include fixed dose subcutaneous formulations of pertuzumab and subcutaneous co-formulations of pertuzumab and trastuzumab, and their use in the treatment of cancer.
Owner:F HOFFMANN LA ROCHE & CO AG
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20160175438A1Prolong progression-free survivalReduce riskOrganic active ingredientsAntibody ingredientsHER2 Positive Breast CancerProgression-free survival
The present application describes uses for Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; and treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag.
Owner:GENENTECH INC
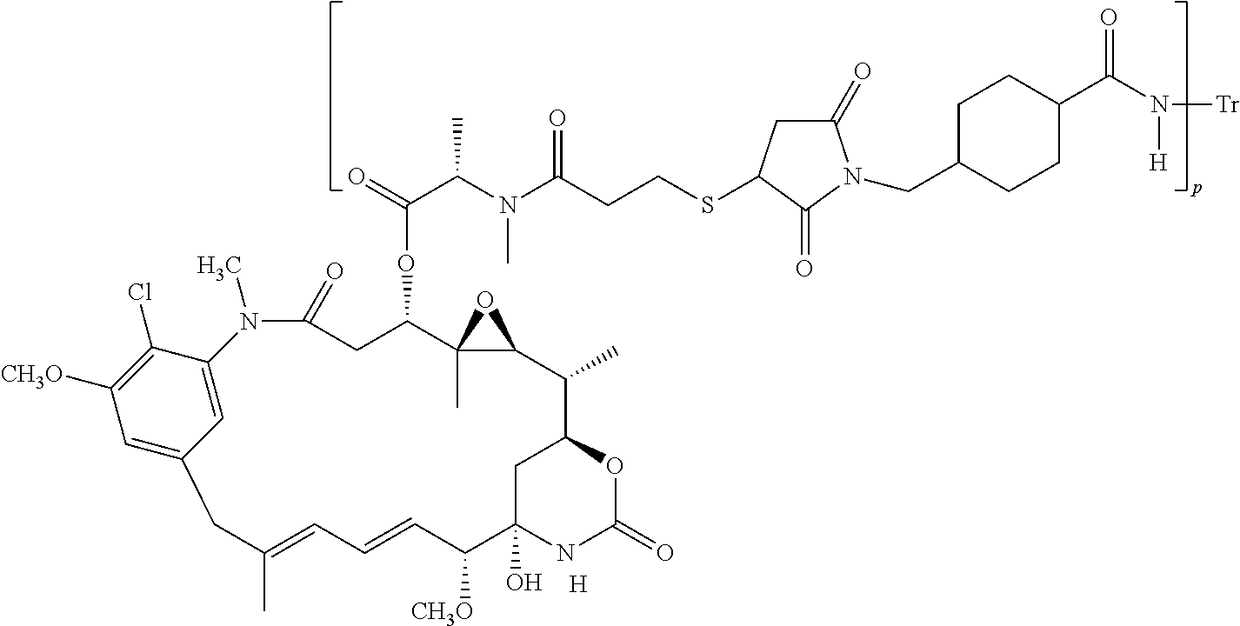
Antibody-drug conjugate Pertuzumab-MCC-DM1, conjugate and Trastuzumab composition, and application of conjugate and composition
InactiveCN104892763AGood treatment effectOrganic active ingredientsAntibody ingredientsCancer cellTherapeutic effect
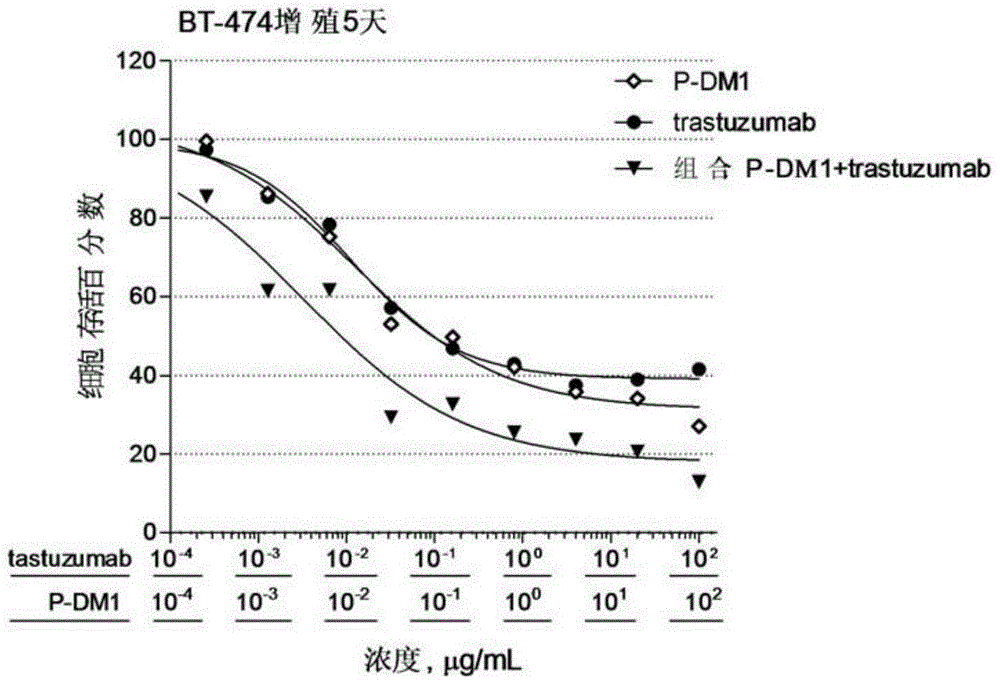
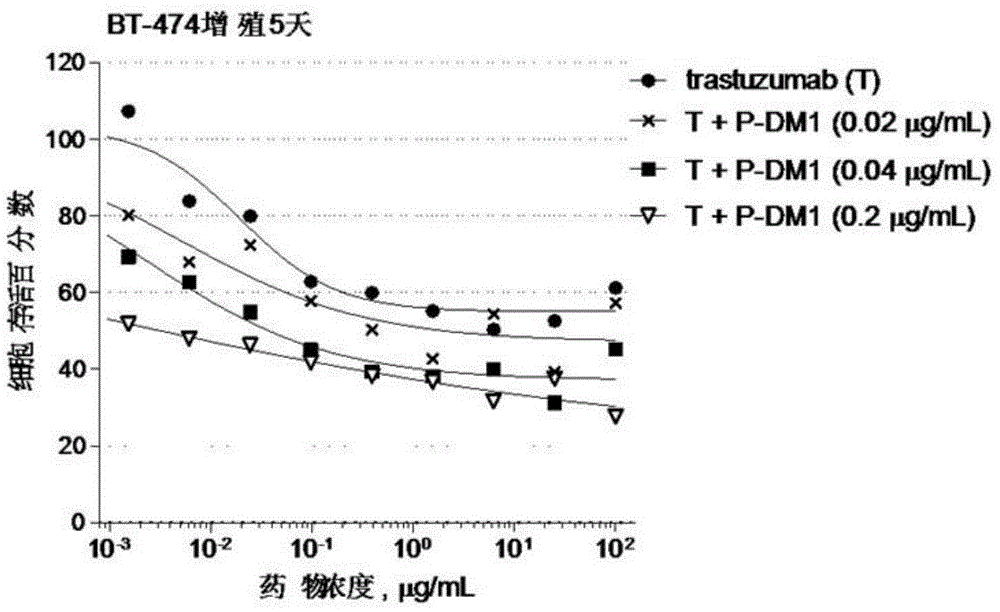
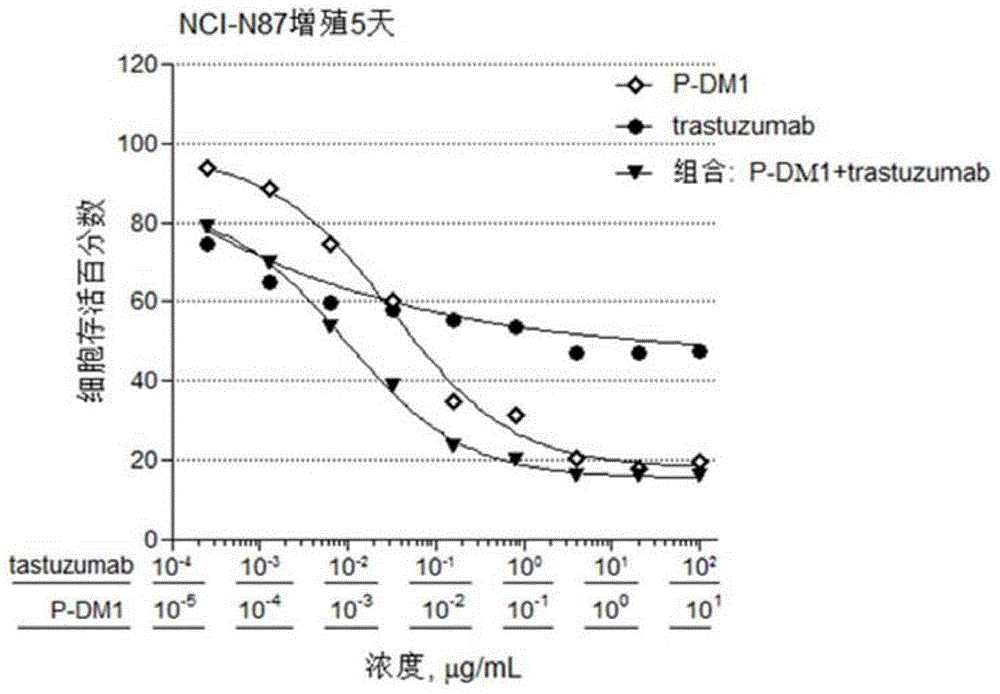
Provided are antibody-drug conjugate Pertuzumab-MCC-DM1 (that is, P-DM1) and a treating agent composition comprising the P-DM1 and an HER2 dimerisation inhibitor antibody. Also provided are uses of the P-DM1 and the treating agent composition in treating highly proliferative diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Treatment of her2-positive breast cancer
InactiveUS20180134803A1Improve responseSignificant increase in in adverse eventsOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHER2 Positive Breast CarcinomaAnthracycline
Methods for the treatment of HER2-positive breast cancer are provided by neoadjuvant administration of pertuzumab and trastuzumab in combination with anthracycline-based chemotherapy. In particular, the methods concerns the treatment patients with HER2-positive, locally advanced, inflammatory, or early-stage breast cancer by neoadjuvant administration of pertuzumab and trastuzumab following anthracycline-based chemotherapy, wherein the combined administration of pertuzumab and trastuzumab increases pathological complete response (pCR) relative to administration of trastuzumab as a single agent, without significant increase in adverse events, such as cardiac toxicity, relative to neoadjuvant anthracycline-based chemotherapy.
Owner:F HOFFMANN LA ROCHE & CO AG
Specific HER2 protein targeted polypeptide and application thereof
ActiveCN105693860AHigh puritySmall molecular weightRadioactive preparation carriersImmunoglobulinsChemical synthesisCancer cell
The invention provides specific HER2 (human epidermal growth factor receptor 2) protein targeted polypeptide. The polypeptide is a mutant obtained with pertuzumab 46-65th amino acid as a template, can be specifically combined with HER2 high-expression positive breast cancer cells, and has the higher affinity than pertuzumab. The invention further provides a derivative of the polypeptide. The derivative is a bivalent or polyvalent formed from the polypeptide. The polypeptide has the character of targeting HER2 positive cancer cells, and is high in selectivity. The polypeptide can be prepared through a chemical synthesis method, is high in purity, small in molecular weight, strong in specificity, free of immunogenicity, safe and reliable, and can be used for cancer targeted therapy or cancer targeted molecular imaging and diagnosis.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Treatment of advanced her2 expressing cancer
InactiveUS20180221481A1High response rateIncreased partial responseAntibody ingredientsImmunoglobulinsPancreasOncology
Methods for the treatment of patients with HER2-positive, HER2-amplified and / or HER2-mutated advanced cancer by administration of pertuzumab plus trastuzumab are disclosed. In one aspect, the cancer is advanced HER2-positive, HER2-amplified and / or HER2-mutated colorectal, biliary, bladder, urothelial, salivary, lung, pancreatic, ovarian, prostate, or skin cancer. In another aspect, the cancer is HER2-positive, HER2-amplified and / or HER2-mutated colorectal, biliary, bladder, urothelial, salivary, lung, pancreatic, ovarian, prostate, or skin cancer that is refractory to one or more other treatment regimens.
Owner:GENENTECH INC
Extending survival of cancer patients with elevated levels of egf or tgf-alpha
The present application describes extending survival in a cancer patient, where the patient is producing an elevated level of EGF or TGF-alpha, by treating the patient with a HER dimerization inhibitor, such as pertuzumab.
Owner:GENENTECH INC +1
Treatment of advanced her2 expressing cancer
InactiveUS20200237910A1High response rateImprove responseAntibody ingredientsImmunoglobulinsProstate cancerBiliary tract
Methods for the treatment of patients with HER2-positive, HER2-amplified and / or HER2-mutated advanced cancer by administration of pertuzumab plus trastuzumab are disclosed. In one aspect, the cancer is advanced HER2-positive, HER2-amplified and / or HER2-mutated colorectal, biliary, bladder, urothelial, salivary, lung, pancreatic, ovarian, prostate, or skin cancer. In another aspect, the cancer is HER2-positive, HER2-amplified and / or HER2-mutated colorectal, biliary, bladder, urothelial, salivary, lung, pancreatic, ovarian, prostate, or skin cancer that is refractory to one or more other treatment regimens.
Owner:GENENTECH INC
Antibody for resisting human epidermal growth factor receptor 2 (HER2), as well as medical composition and use of antibody
InactiveCN107446045AGood curative effectGood tumor suppressor effectCompounds screening/testingImmunoglobulins against cell receptors/antigens/surface-determinantsHigh cellAntigen
The invention belongs to the field of tumor treatment and the field of molecular immunology, and relates to an antibody for resisting human epidermal growth factor receptor 2 (HER2), as well as a medical composition and use of antibody, in particular to the antibody for resisting Her2 or an antigen binding fragment of the antibody for resisting Her2. The antibody for resisting Her2 comprises a first heavy chain and a second heavy chain, wherein according to the first heavy chain, a variable region of heavy chain (VH) of the first heavy chain comprises a complementarity-determining region (CDR) of which the amino acid sequence is SEQ ID No: 1-3, and the amino acid sequence of the CH of the first heavy chain is as shown in SEQ ID No: 7; according to the second heavy chain, a variable region of heavy chain (VH) of the second heavy chain comprises a complementarity-determining region (CDR) of which the amino acid sequence is SEQ ID No: 4-6, and the amino acid sequence of the CH of the first heavy chain is as shown in SEQ ID No: 8. Compared with the combination of two antibodies of trastuzumab and pertuzumab, the antibody disclosed by the invention has higher cell direct killing activity and higher antibody dependent cellmediated cytotoxicity (ADCC) activity; the complement-dependentcytotoxicity (CDC) activity of the two antibodies of trastuzumab and pertuzumab is equivalent to that of the antibody disclosed by the invention; the antibody disclosed by the invention adopts a structure of a normal antibody; and fucose is also knocked out, so that the ADCC activity is improved.
Owner:BEIJING MABWORKS BIOTECH
Pertuzumab plus trastuzumab fixed dose combination
PendingUS20210403599A1Easy to manageOvercome inconveniencePeptide/protein ingredientsAntibody ingredientsTrial drugEfficacy
The invention disclosed concerns a fixed dose combination (FDC) of pertuzumab, trastuzumab, and, optionally, recombinant human hyaluronidase (rHuPH20), which is administered subcutaneously to patients. The final efficacy and safety data for the FeDeriCa clinical trial, United States Prescribing Information (USPI) (including home-use) methods, and primary analysis of the PHranceSCa clinical trial are disclosed and claimed.
Owner:GENENTECH INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com