Antibody composition preparation and application thereof
A technology of antibody composition and preparation, which is applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc., to achieve the effects of long-term storage, good tumor treatment activity, and prevention of content increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Liquid and lyophilized pharmaceutical product formulations for parenteral administration according to the invention are as follows:
[0056] 1. Preparation of liquid preparations
[0057] The solutions of Trastuzumab and Pertuzumab were exchanged into the corresponding preparation buffer (such as 20mM L-histidine, pH 6.0 or 20mM L-histidine, 0.02 %w / v polysorbate 20, pH6.0), and concentrated to a certain protein concentration, such as 70mg / ml. It is then diluted to the desired protein concentration with the corresponding formulation buffer. Protein-stabilizing formulation components such as sucrose, amino acids such as L-arginine can be added in dissolved or solid form, and surfactants can be added in stock solution form. Finally, the Trastuzumab and Pertuzumab preparation solutions of the same volume were mixed evenly. All formulations were filter sterilized through a 0.22 μm filter, then aseptically dispensed into sterile glass vials and sealed with rubber stoppers...
Embodiment 2
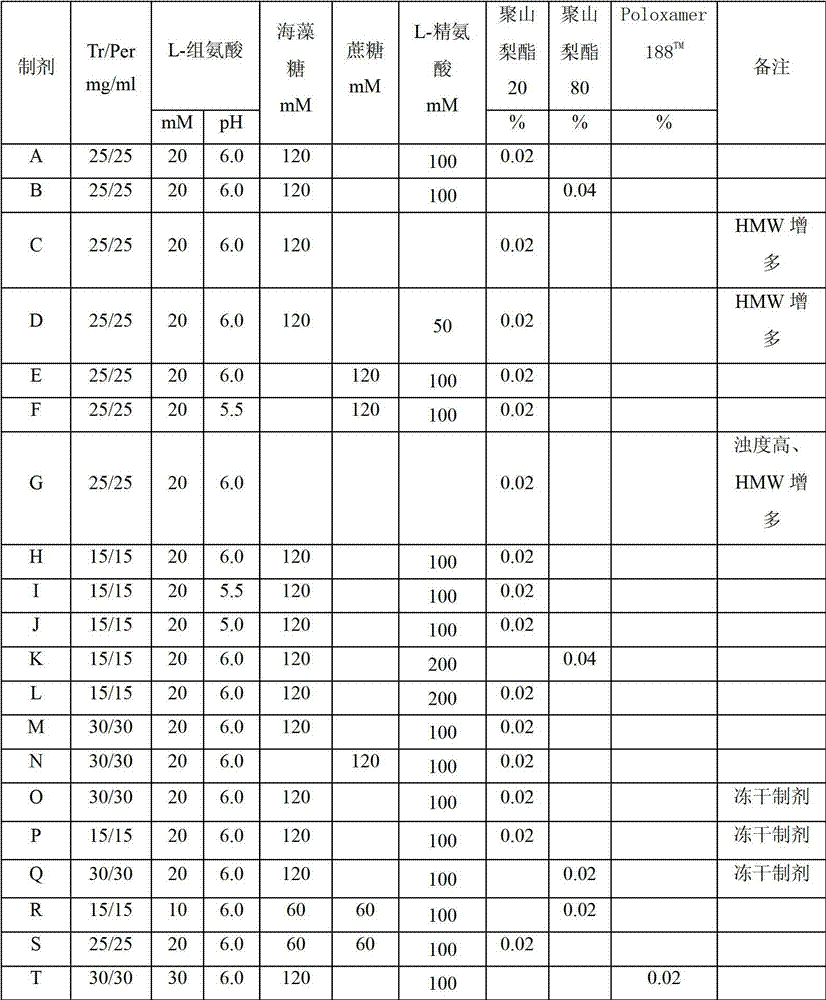
[0072] Example 2: Formulation A
[0073] Formula: 25mg / ml Trastuzumab, 25mg / ml Pertuzumab, 20mM L-histidine, 120mM trehalose, 100mM L-arginine, 0.02%Tween20, pH6.0;
[0074] Table 3: Stability data for Formulation A
[0075]
Embodiment 3
[0076] Example 3: Formulation B
[0077] Formula: 25mg / ml Trastuzumab, 25mg / ml Pertuzumab, 20mM L-Histidine, 120mM Trehalose, 100mM L-Arginine, 0.04%Tween80, pH6.0;
[0078] Table 4: Stability data for Formulation B
[0079]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com