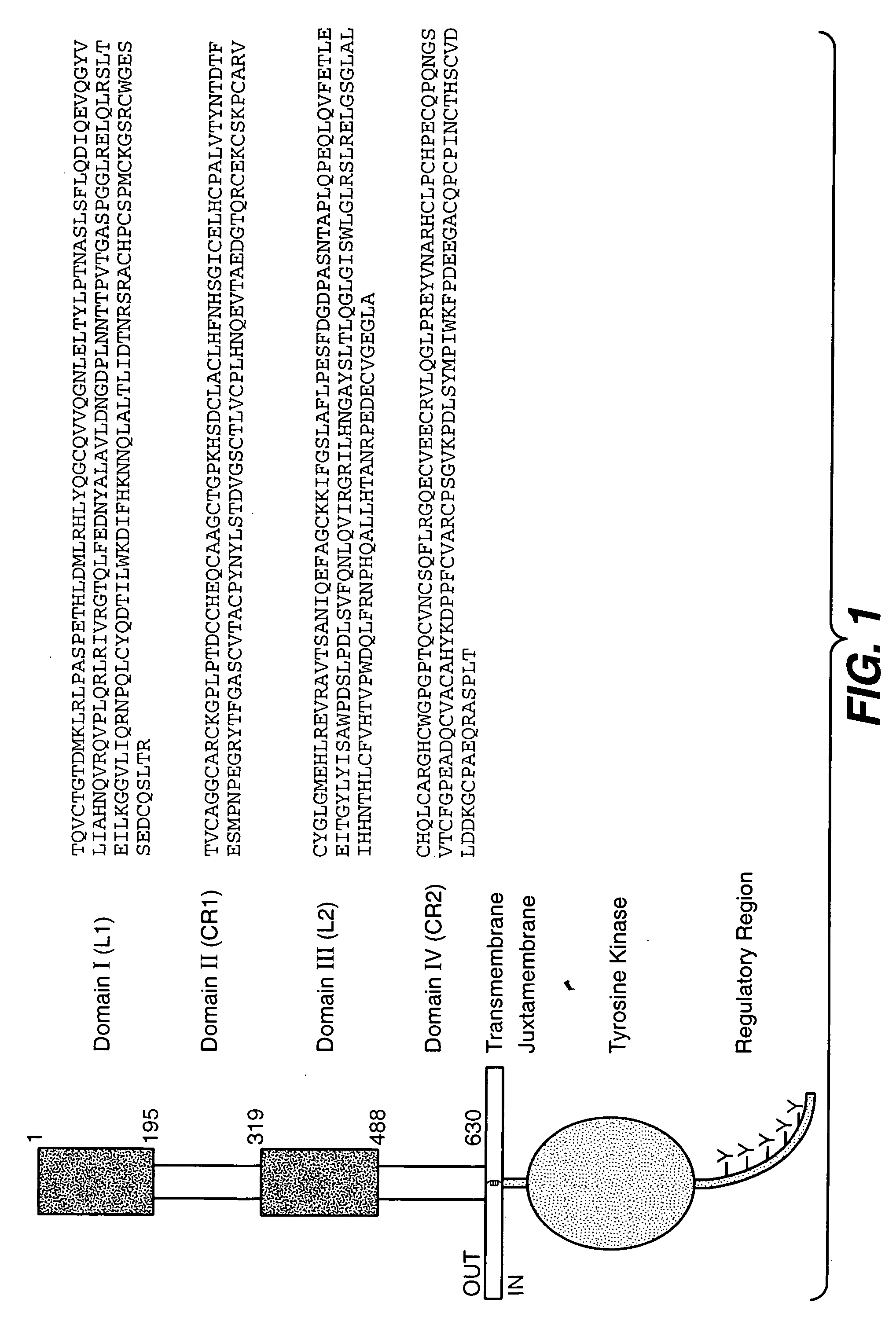
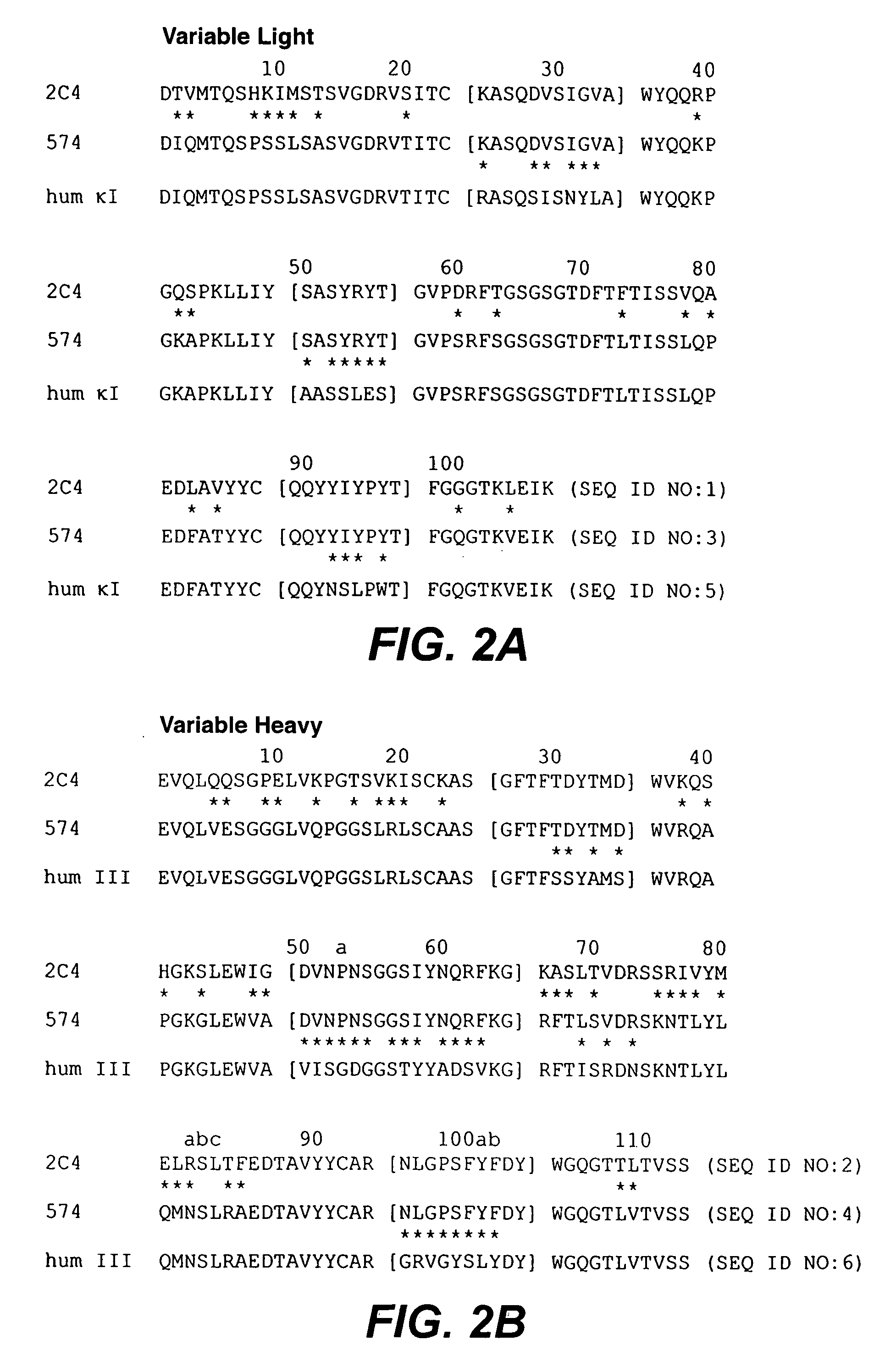
Extending time to disease progression or survival in cancer patients
a cancer patient and disease technology, applied in the field of extending time to disease progression or survival in a cancer patient, can solve the problems of reducing tumor proliferation and survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Clinical Activity of Pertuzumab in Advanced, Refractory or Recurrent Ovarian Cancer and the Role of HER2 Activation Status
[0358] This example concerns a single arm, open label, multicenter phase II clinical trial of ovarian cancer patients. Patients with advanced, refractory or recurrent ovarian cancer were treated with pertuzumab, a humanized HER2 antibody. Pertuzumab represents a new class of targeted agents called HER dimerization inhibitors (HDIs) that inhibit dimerization of HER2 with EGFR, HER3 and HER4, and inhibit signaling through MAP and P13 kinase.
[0359] 65 patients with relapsed ovarian cancer were enrolled with 61 receiving therapy with “low dose” single agent pertuzumab; pertuzumab was administered intravenously (IV) with a loading of 840 mg followed by 420 mg every 3 weeks.
[0360] A second cohort of patients was treated with “high dose” pertuzumab; 1050 mg every 3 weeks, administered as a single agent. In this cohort, 64 subjects were enrolled, with 62 subjects bein...
example 2
Phospho-HER2 ELISA for Determining HER2 Activation
[0372] Example 1 above describes the clinical trial which evaluated the efficacy of pertuzumab in subjects with advanced, refractory or recurrent ovarian cancer. This example describes development of the assay used to determine HER2 activation in the patients treated in Example 1.
[0373] The phospho-HER2 ELISA was developed to measure the concentration of HER2-associated tyrosine phosphorylation (HER2 / pTyr) in human ovarian tumor tissue lysates. The assay utilizes COSTAR™ 96-well, half-area, microtiter plates because of limited sample volume. The coat antibody is an affinity purified goat anti-HER2 ECD and the secondary antibody is a biotinylated murine monoclonal (clone 4G10) specific for phosphotyrosine. The reference standard is a SK-BR-3 cell lysate with an assay range of 132 U / mL. One unit equals the amount of phosphorylated tyrosine measured in a SK-BR-3 cell lysate containing 277 pg total HER2 as determined by the Total HER2 ...
example 3
Gene Expression Profiling for Determining HER2 Activation
[0421] This example shows how HER2 activation can be evaluated by determining gene expression profiles as an alternative to determining HER2 phosphorylation directly. This profiling may be done on fresh, frozen, or formalin-fixed, paraffin-embedded ovarian tumor specimens, but preferably the latter.
[0422] Ovarian cancer specimens treated with pertuzumab were profiled for gene expression using AFFYMETRIX® microarray analysis performed according to the manufacturer's instructions. The microarray expression data was analyzed to identify gene patterns which would be associated with HER2 phosphorylation status. Remarkably, a pattern emerged where tumors with relatively high levels of expression of EGFR, HER2, HER3, and the HER ligand betacelullin were also positive for HER2 phosphorylation. The correlation was positive in six of the six HER2 phosphorylation positive cases, and none of the HER2 phosphorylation negative cases were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com