Antibody-drug conjugate Pertuzumab-MCC-DM1, conjugate and Trastuzumab composition, and application of conjugate and composition
A P-DM1, drug conjugate technology, applied in the field of antibodies, can solve the problems of apoptosis, growth retardation, etc., achieve good therapeutic effect, and improve the effect of therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of Pertuzumab-MCC-DM1 (P-DM1)
[0057] The preparation steps are as follows:
[0058] 1) Buffer exchange of antibodies
[0059] Use desalting chromatography (SephadexTM G25 desalting column) to replace the Pertuzumab monoantigen solution into the reaction buffer (50mM potassium phosphate / 50mM NaCl / 2mM EDTA, pH7.5), and concentrate the antibody concentration to 5mg / ml to prepare Pertuzumab antibody;
[0060] 2) Preparation of MCC-DM1 mother liquor
[0061] Weigh MCC-DM1 and fully dissolve it with dimethylacetamide (DMA) to prepare 10mg / ml MCC-DM1 mother solution;
[0062] 3) Coupling reaction
[0063] Add the MCC-DM1 master solution prepared in step (2) to the pertuzumab prepared in step (1) for conjugation reaction. Antibodies are initially titrated with several excesses of MCC-DM1 to determine the desired DM1:mAb ratio, typically in the range of 6-10 fold molar excess for human antibodies. DMA was within 5% v / v in the reaction. The reaction ...
Embodiment 2
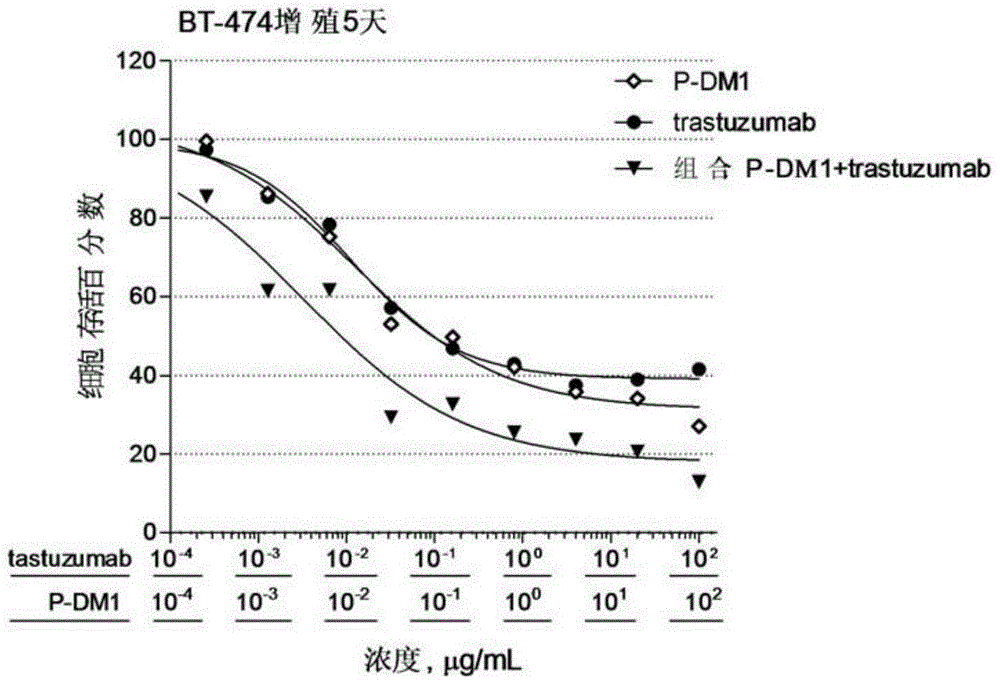
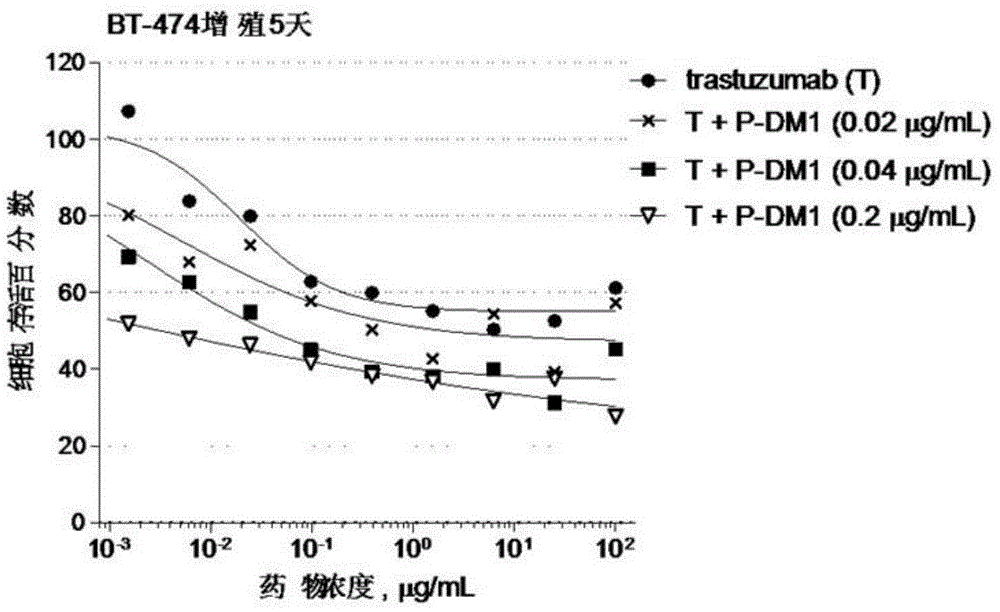
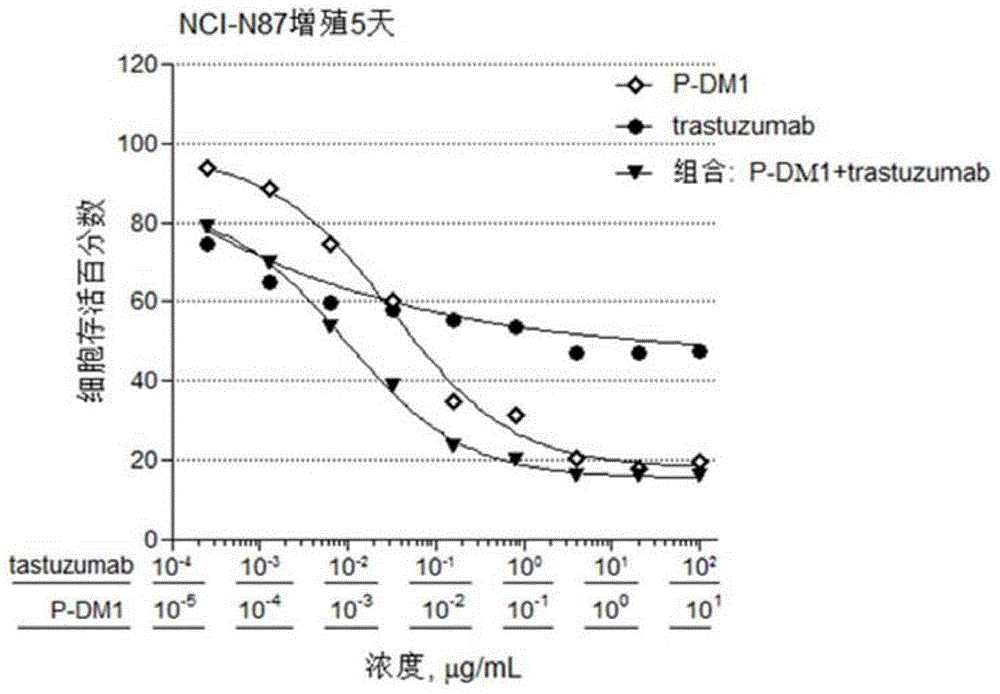
[0075] Embodiment 2: in vitro cell proliferation assay biological activity experiment
[0076] The experimental materials used in the following experiments were obtained from: DMEM medium, F12K medium, RPMI1640 medium, 0.25% trypsin-EDTA, fetal bovine serum, 100× sodium pyruvate, and 100× penicillin streptomycin were purchased from Gibco. Sulforhodamine B (Sulforhodamine B, SRB) was purchased from Sigma. BT-474 breast cancer cells and NCI-N87 gastric cancer cells were from Kunming Cell Bank, Chinese Academy of Sciences, SK-BR-3 breast cancer cells were from Shanghai Bogu Biotechnology Co., Ltd., and Calu-3 lung cancer cells were from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences Banks, SKOV-3 ovarian cancer cells and DU-145 prostate cancer cells were from the American Type Culture Collection (ATCC). All other reagents were analytically pure. 96-well flat bottom polystyrene (Corning, catalog #3599). Synergy2 microplate reader (Bio-Tek).
[0077] I...
Embodiment 3
[0084] Embodiment 3: In vivo anti-tumor efficacy assay experiment:
[0085] The efficacy of the combinations of the invention can be measured in vivo by implanting allografts or xenografts of cancer cells in rodents and treating tumors with the combination. Test mice are treated with drug or control and monitored for several weeks or longer to measure time to tumor doubling, log cell kill, and tumor inhibition.
[0086] 1) Experimental animals
[0087] BALB / cA-nude nude mice, 6-7 weeks old, male, were purchased from Shanghai Slack Experimental Animal Co., Ltd. Certificate number: SCXK (Shanghai) 2012-0002. Breeding environment: SPF grade.
[0088] 2) Experimental steps
[0089]Nude mice were subcutaneously inoculated with human breast cancer BT-474 / T721 cells, and after the tumor grew to 100-200mm3, the animals were randomly divided into groups (D0). See Table 2 for dosage and regimen. The tumor volume was measured twice a week, the mice were weighed, and the data were r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com