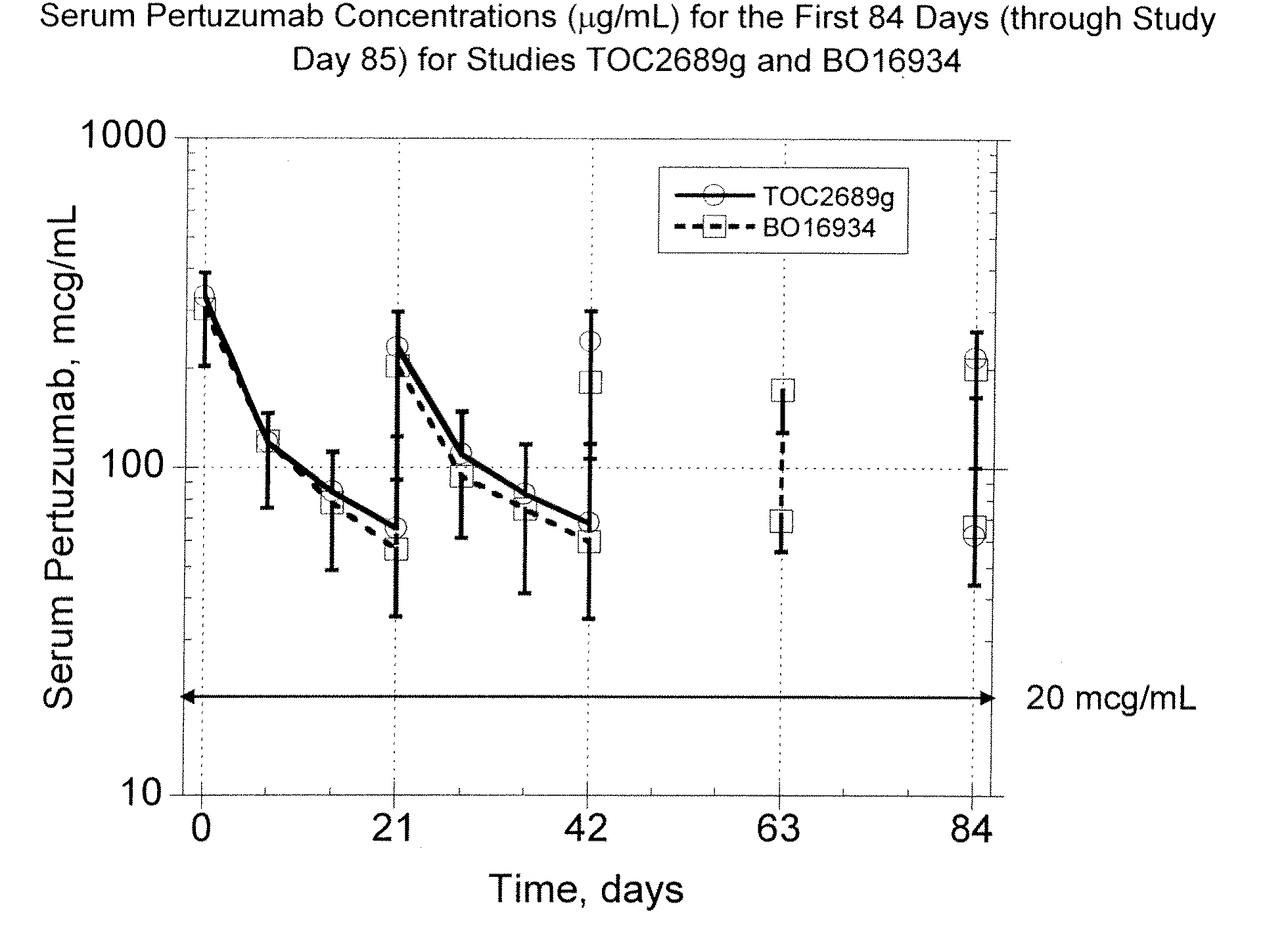
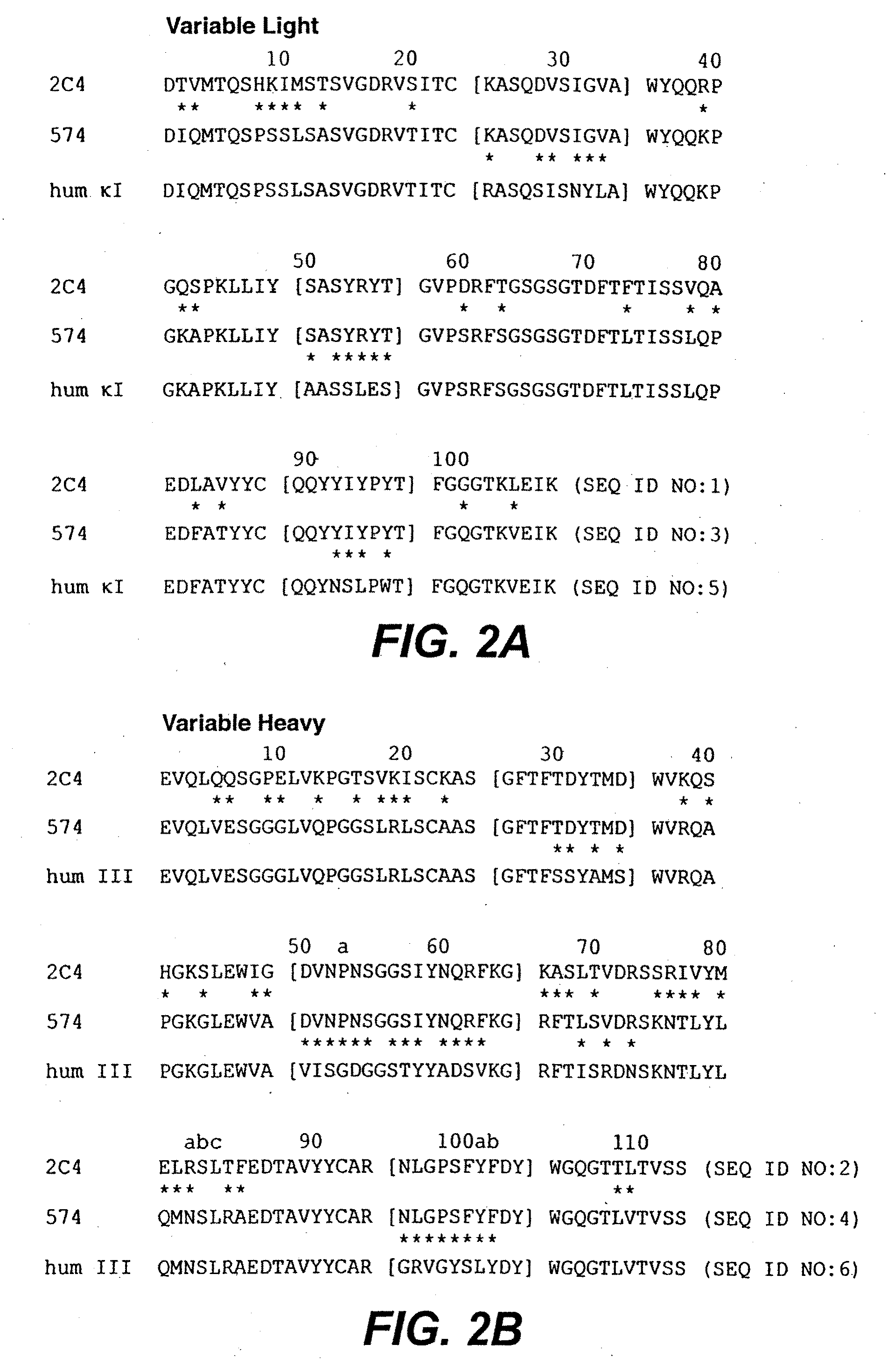
Treatment of metastatic breast cancer
a breast cancer and metastatic technology, applied in the field of metastatic breast cancer treatment, can solve the problems of short-lived rare complete responses, reduced tumor proliferation and survival, etc., and achieve the effect of prolonging the survival of human patients, prolonging progression free survival (pfs) or overall survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Phase III Clinical Study to Evaluate the Efficacy and Safety of Pertuzumab+Trastuzumab+Docetaxel Treatment of Previously Untreated Metastatic Breast Cancer
[0356]Primary Objectives
[0357]The primary objective of this study is to compare progression-free survival (PFS) based on tumor assessments by an independent review facility (IRF) between patients in two treatment arms:
[0358]placebo+trastuzumab+docetaxel vs. pertuzumab+trastuzumab+docetaxel.
[0359]Secondary Objectives[0360]To compare overall survival (OS) between the two arms[0361]To compare PFS between the two treatment arms based upon investigator assessment of progression[0362]To compare the overall objective response rate between the two treatment arms[0363]To compare the duration of objective response between the two treatment arms[0364]To compare the safety profile between the two treatment arms[0365]To compare time to symptom progression, as assessed by the FACT Trial Outcome Index-Physical Functional Breast (TOI-PFB)[0366]To...
example 2
Pharmacokinetic, Drug-Drug Interaction, and QTc Interval Substudy
[0506]This substudy has two main goals: (1) to describe the potential effects of pertuzumab on the QTc interval, and (2) to evaluate the pharmacokinetic profile of pertuzumab in the presence of trastuzumab and docetaxel and to describe any drug-drug interactions that might be observed when all three drugs are co-administered.
[0507]OTC Prolongation
[0508]Drug-induced prolongation of the QT / corrected QT (QTc) interval resulting in increased susceptibility to cardiac arrhythmia is a recognized complication of many drugs across a wide therapeutic spectrum (Morissette et al. Can J Cardiol 2005; 21:857-64). Prolongation of the QT / QTc interval, which is usually asymptomatic, may be manifested by syncope resulting from cardiac arrhythmias such as torsades de pointes (TdP), ventricular arrhythmia, and sudden cardiac death (Morganroth rnst Schering Res Found Workshop 2007; 59:171-84).
[0509]Measurement of QT is made by an electroc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com