Patents
Literature
190 results about "Early breast cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
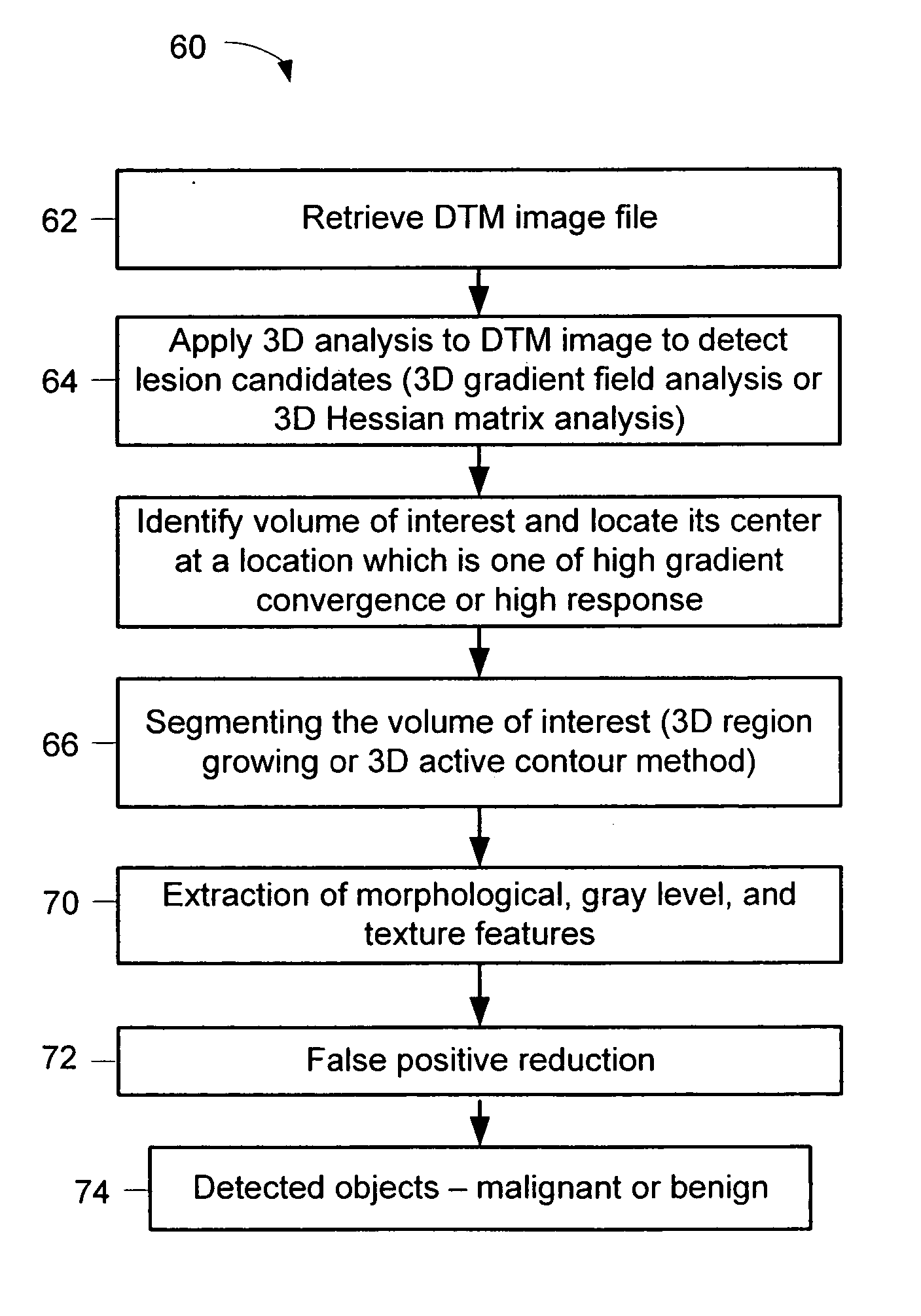
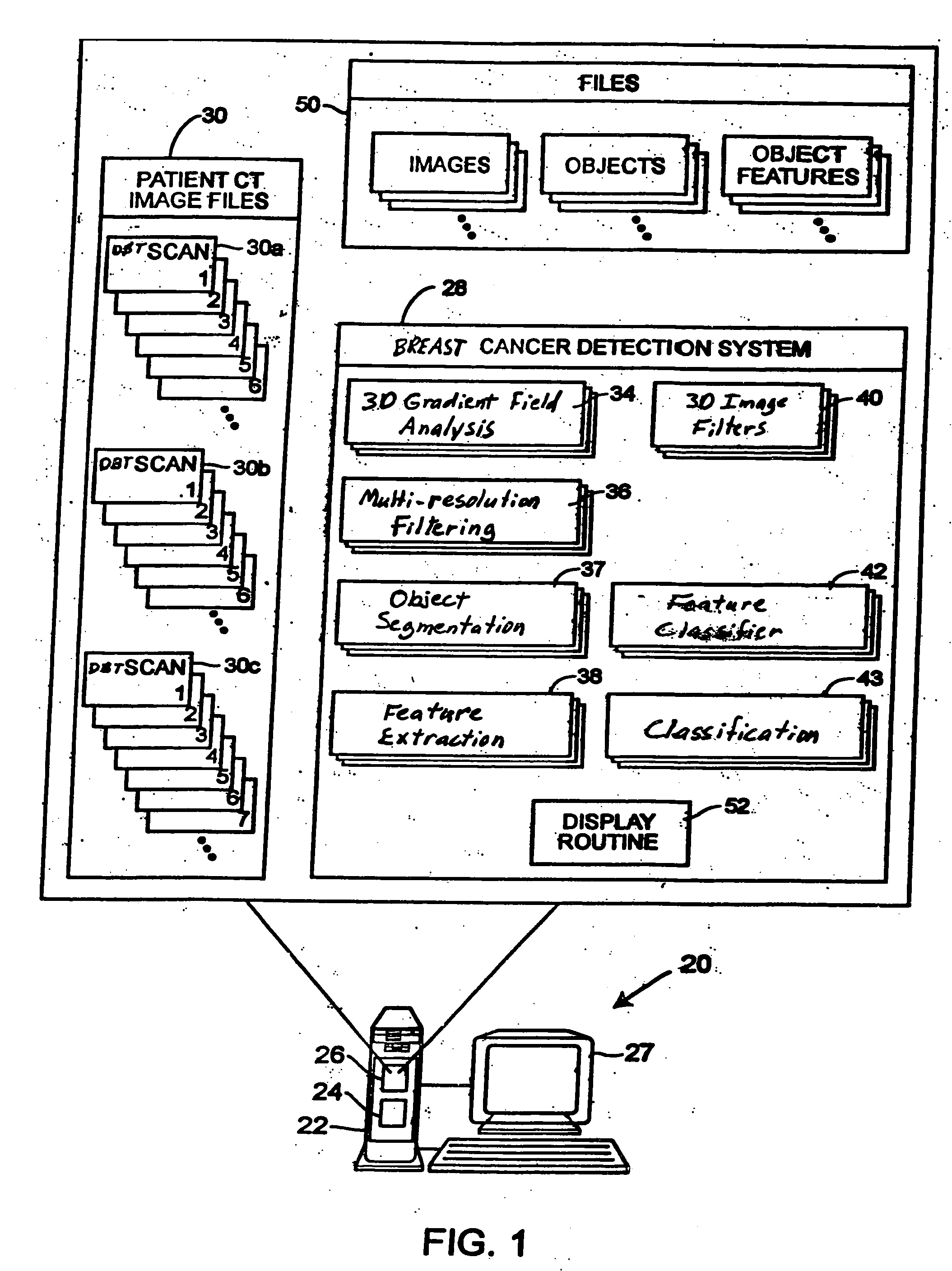
Computerized detection of breast cancer on digital tomosynthesis mammograms
InactiveUS20060177125A1Improve breast cancer detectionExtension of timeImage enhancementImage analysisTomosynthesisGray level
A method for using computer-aided diagnosis (CAD) for digital tomosynthesis mammograms (DTM) including retrieving a DTM image file having a plurality of DTM image slices; applying a three-dimensional gradient field analysis to the DTM image file to detect lesion candidates; identifying a volume of interest and locating its center at a location of high gradient convergence; segmenting the volume of interest by a three dimensional region growing method; extracting one or more three dimensional object characteristics from the object corresponding to the volume of interest, the three dimensional object characteristics being one of a morphological feature, a gray level feature, or a texture feature; and invoking a classifier to determine if the object corresponding to the volume of interest is a breast cancer lesion or normal breast tissue.
Owner:RGT UNIV OF MICHIGAN
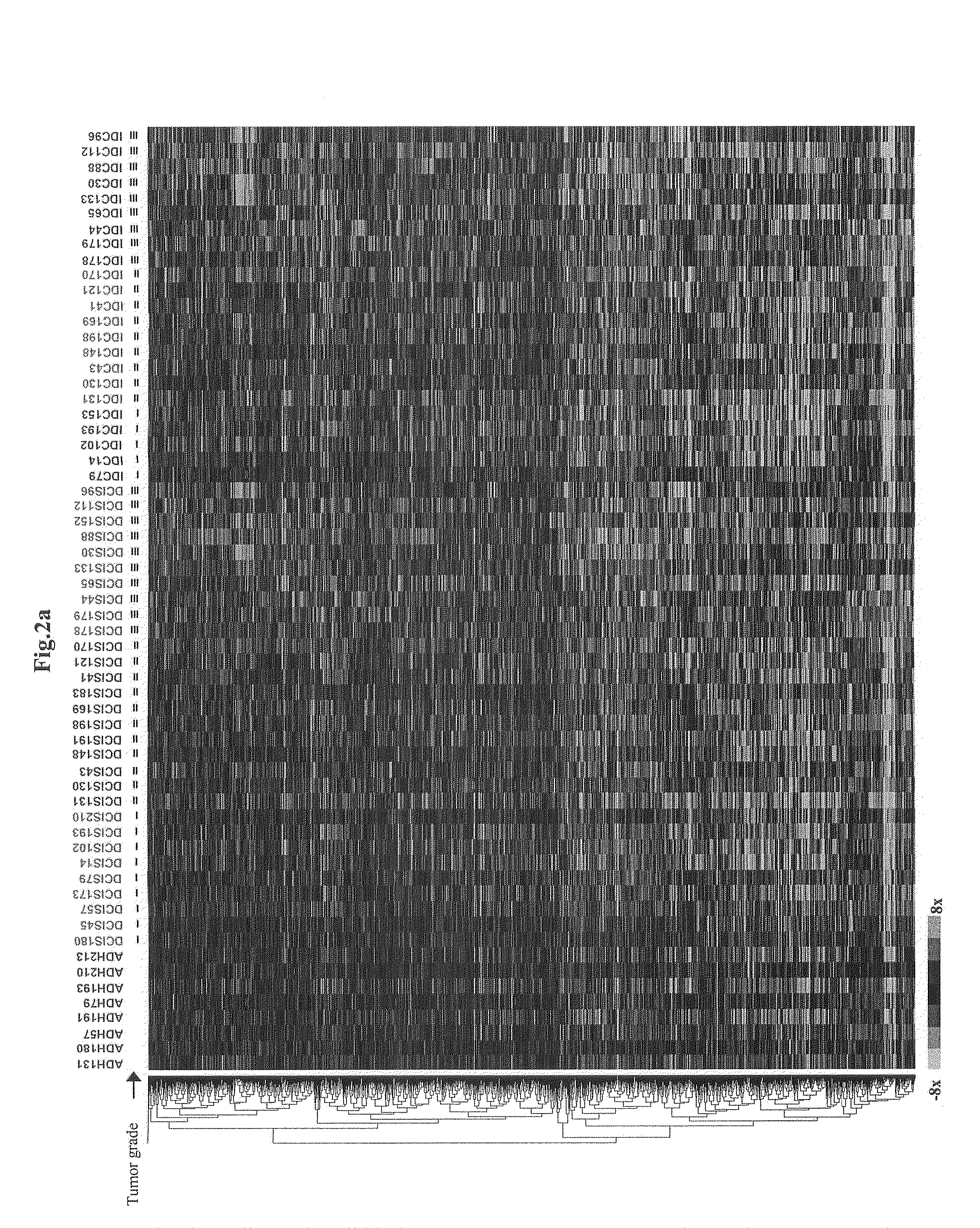
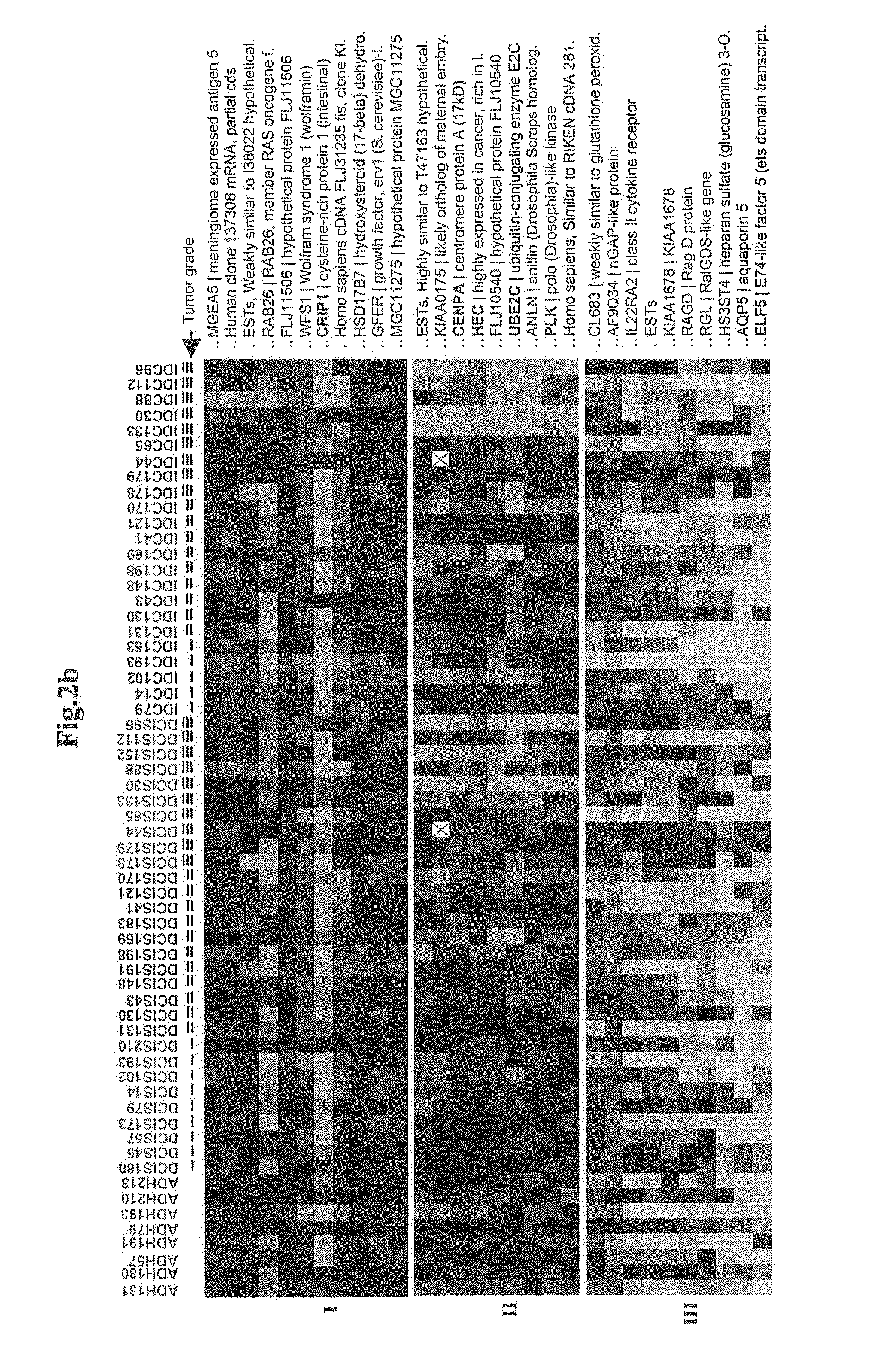
Grading of Breast Cancer
InactiveUS20090092973A1Improve abilitiesAccurate assessmentOrganic active ingredientsSugar derivativesEarly breast cancerOncology
Methods and compositions for the identification of breast cancer grade signatures are provided. The signature profiles are identified based upon multiple sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for identification of cells as being in one or more particular stages and / or grades of breast cancer.
Owner:THE GENERAL HOSPITAL CORP
Ultrasonic treatment of breast cancer
InactiveUS20050038339A1Ultrasonic/sonic/infrasonic diagnosticsUltrasound therapyEarly breast cancerWilms' tumor
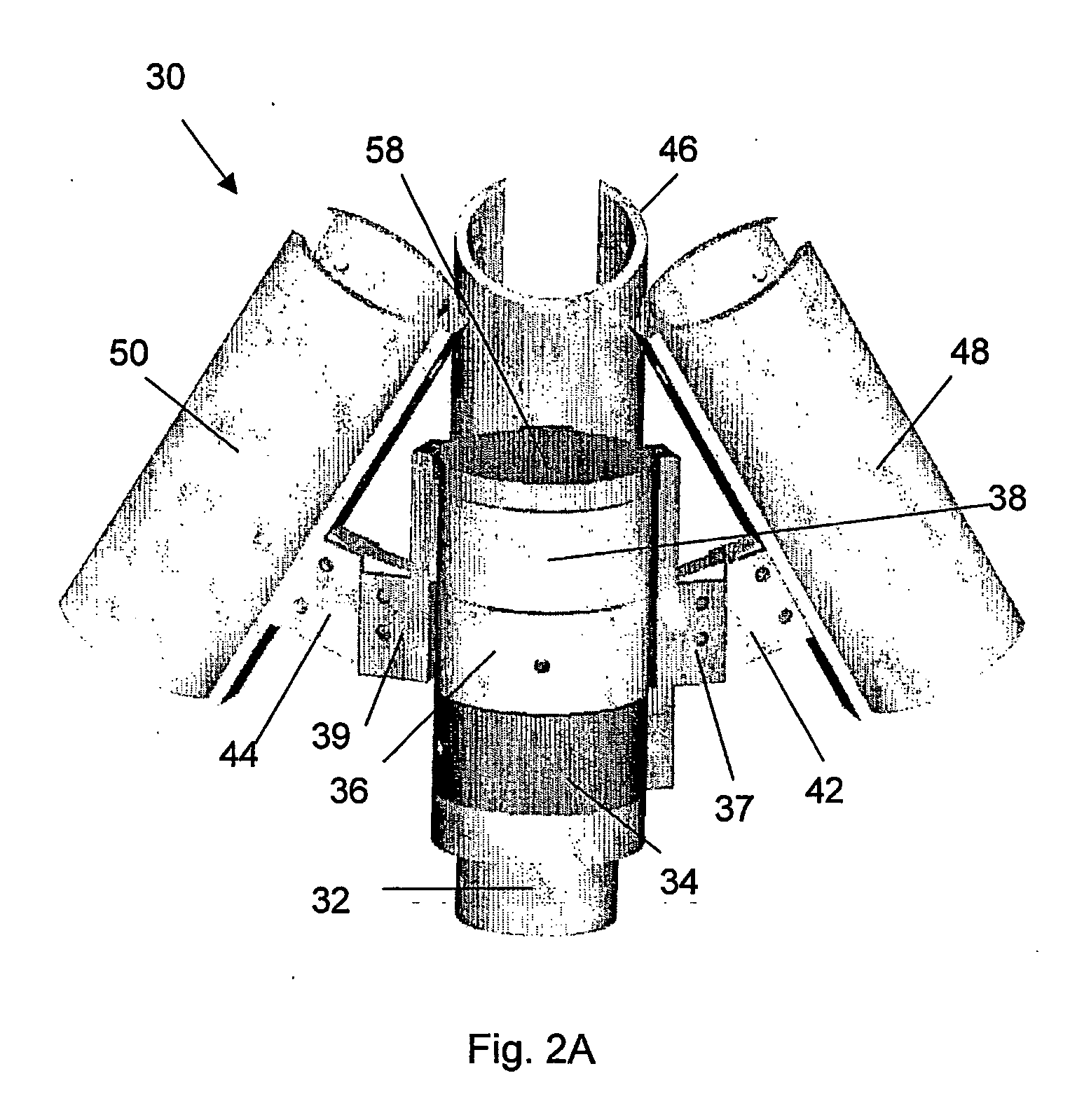
A method of treatment, clinical treatment assembly, robotic manipulator and controlling arrangements for the treatment of cancers are described. The invention has particular application in the treatment of breast cancer. A robotic manipulator (18) carries a jig assembly (30). The jig assembly (30) includes an array of treatment probes (52, 54, 56) and a single identification / diagnostic probe (58). The probes can be moved by the robotic manipulator (18) in three directions (x, y, θ). A subject breast tissue is received in a tank (16) through an operating window (14), and the robotic manipulator (18) is to firstly determine the site of a tumour in the breast tissue. Once the tumour has been located by use of the identification / diagnostic probe (58), the treatment probes (52, 54, 56) are used to ablate the tumour by the superposition of ultrasonic waves at a focal region. A series of such lesions may be performed in sequence to traverse the full extent of the tumour.
Owner:NANYANG TECH UNIV
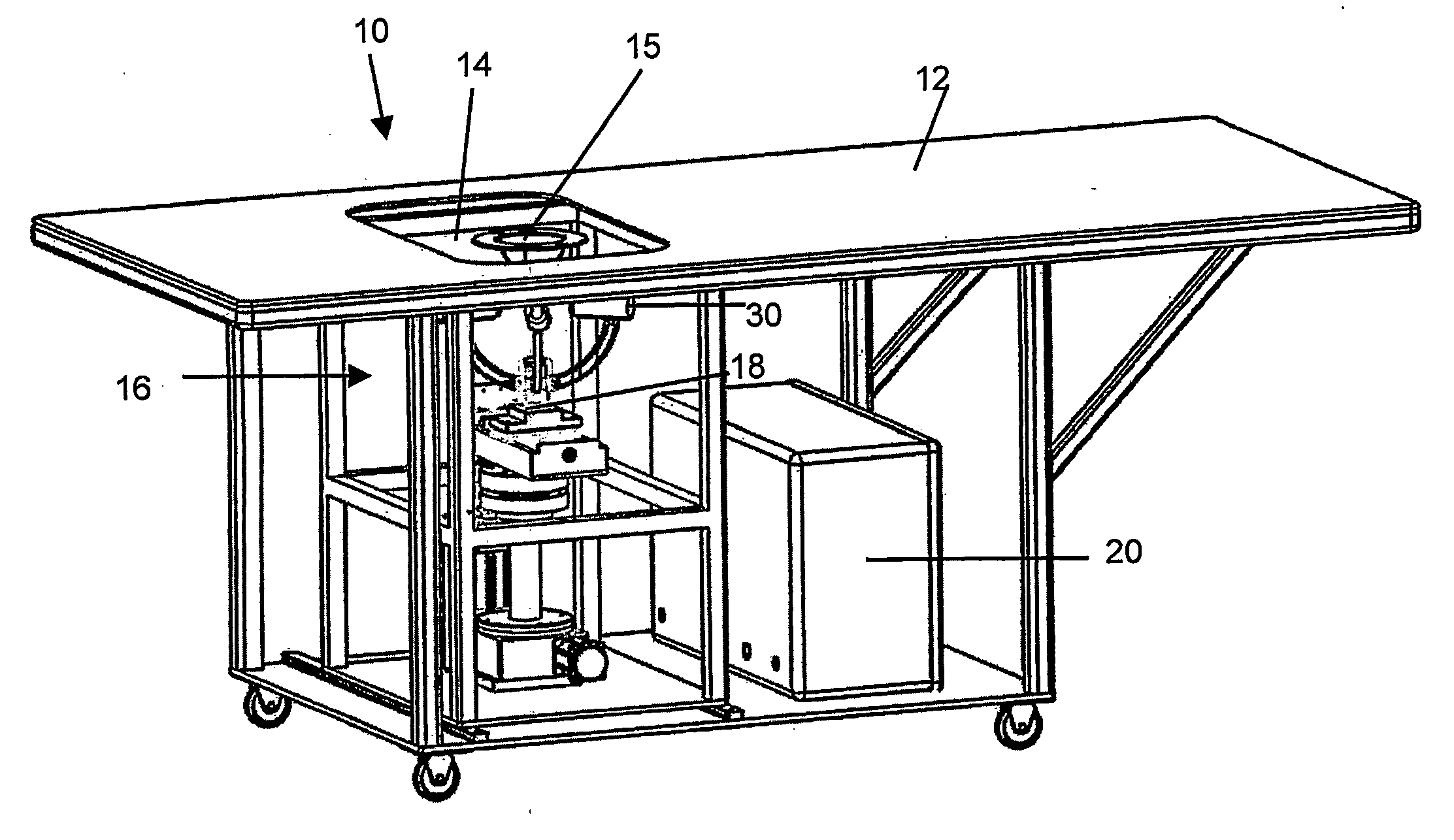
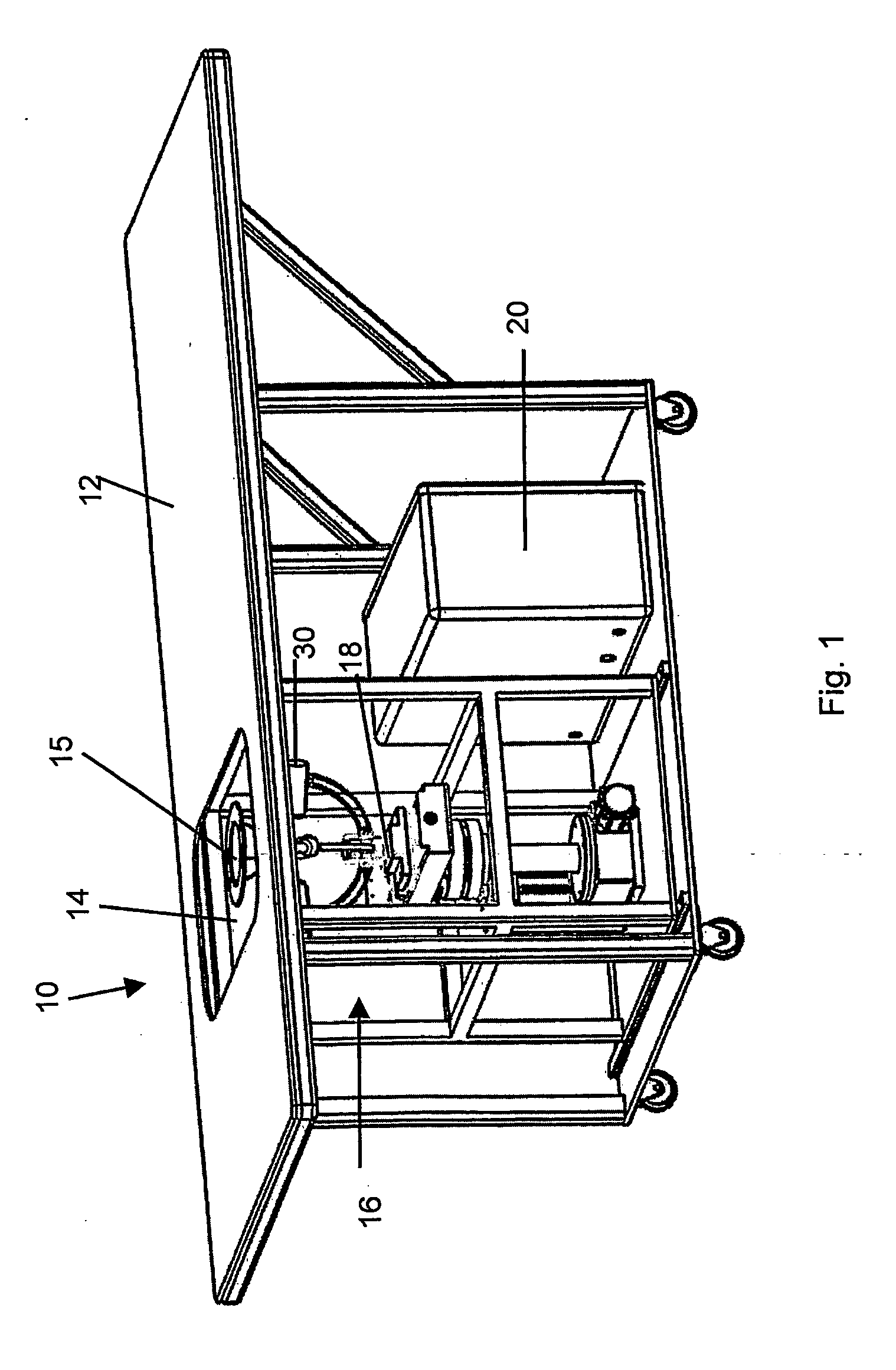
Apparatus and method for diagnosing breast cancer including examination table
ActiveUS8095204B2Safe and simple and comfortable and convenient and effectiveComfortable supportResistance/reactance/impedenceHeart/pulse rate measurement devicesSupporting systemMedicine
A microwave breast cancer imaging method that includes an examination table that is both comfortable and reliable is provided that includes a support system and an orientation system such that breasts can remain in a fixed position to allow for scanning. A horizontal microwave and optically transparent scan plate forms part of the top of the examination table. The imprint of the breasts on the scan plate may be visually displayed to aid in the orienting of each breast such that all volumes within the breast are scanned. Microwave power is then scanned upward through the scan plate to develop a microwave response that is indicative of the presence of a lesion. After scanning, the visual imprint of the breast is recorded. As needed, microwave equipment can be included within a microwave shielded enclosure that also forms part of the scan table. Spurious leakage of microwave power may be further suppressed by use of microwave-absorbing materials, within the enclosure and, in the padding that covers the surface of the examination table and removable pads.
Owner:INTERSTITIAL SYST
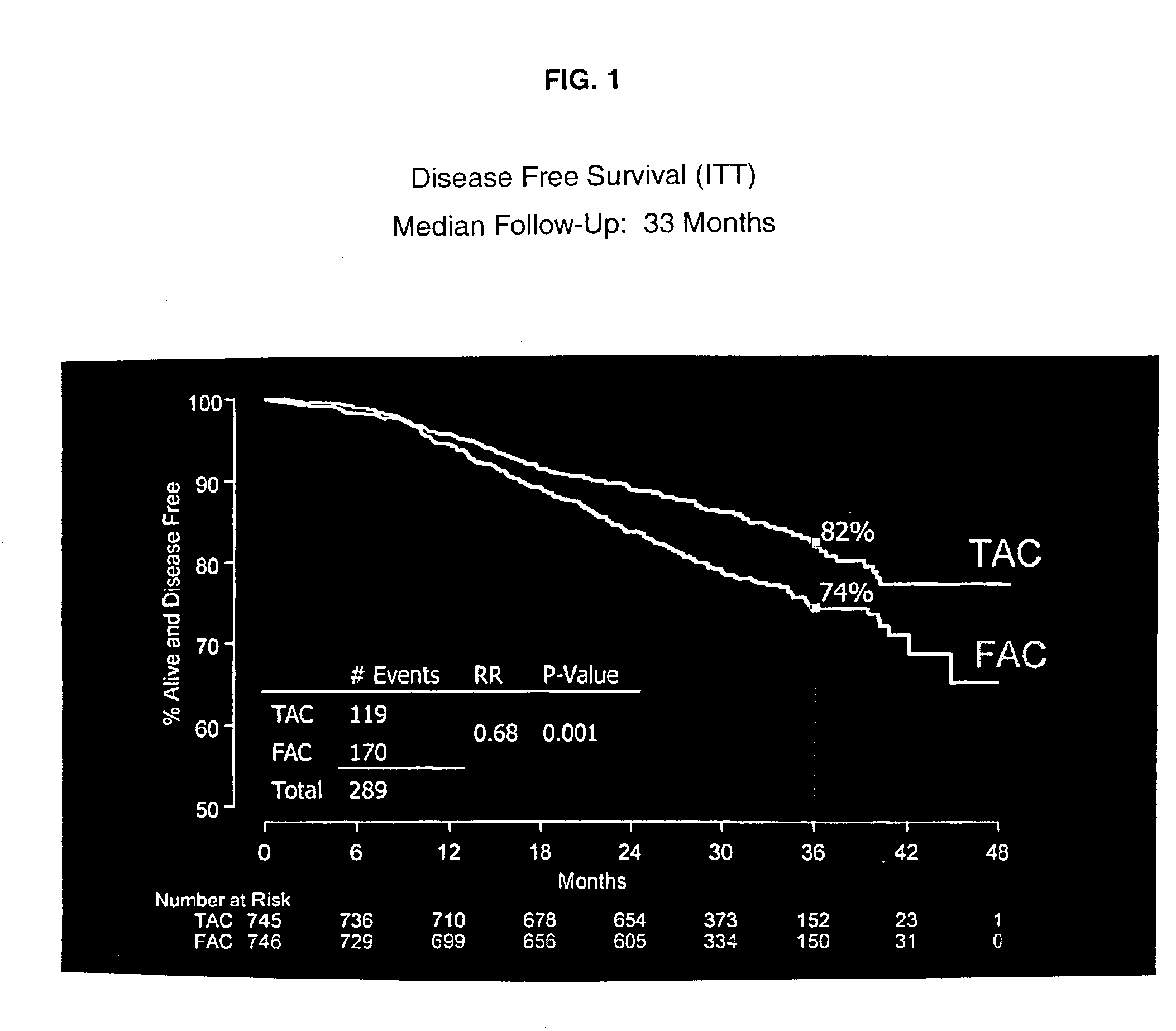
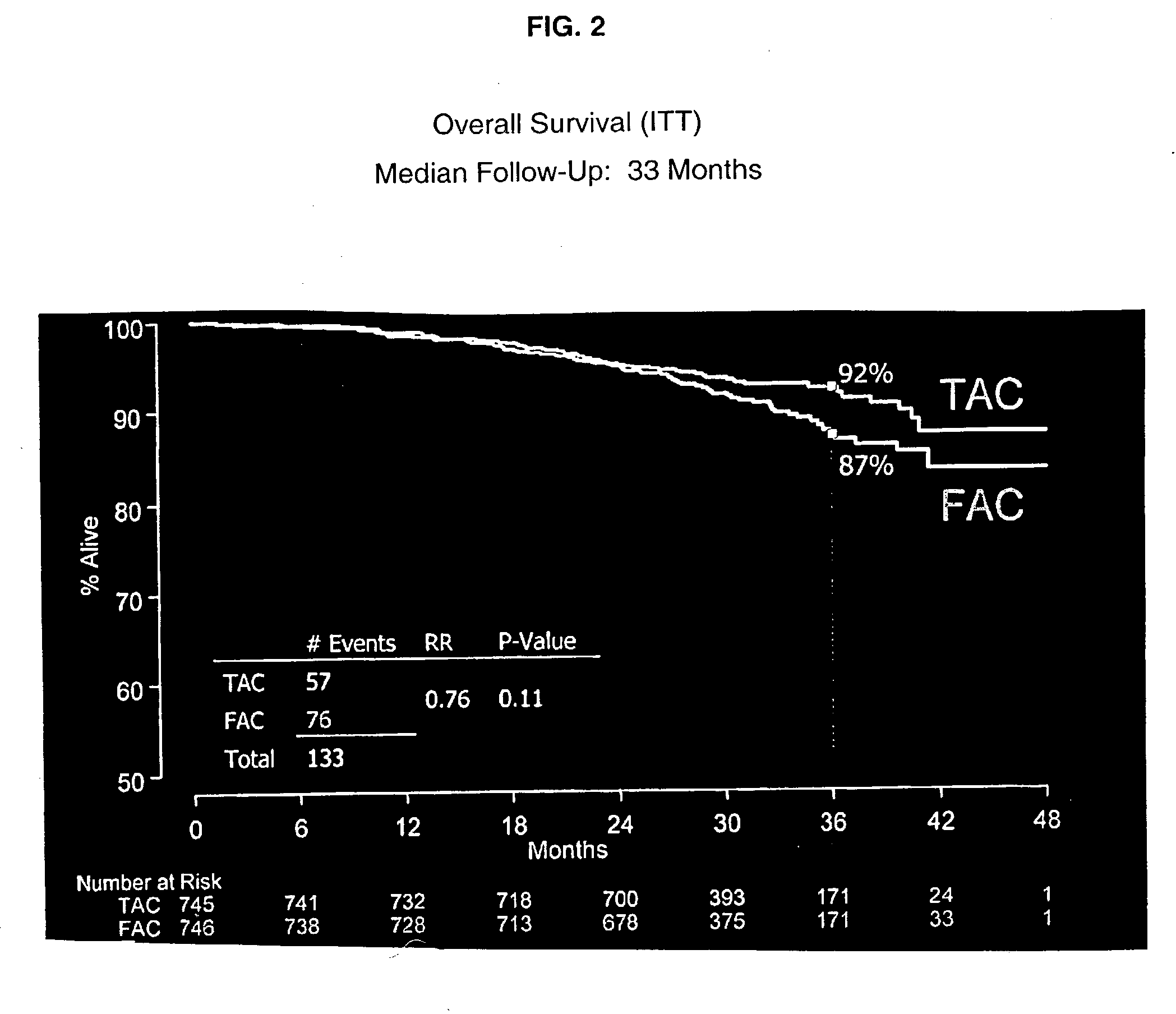
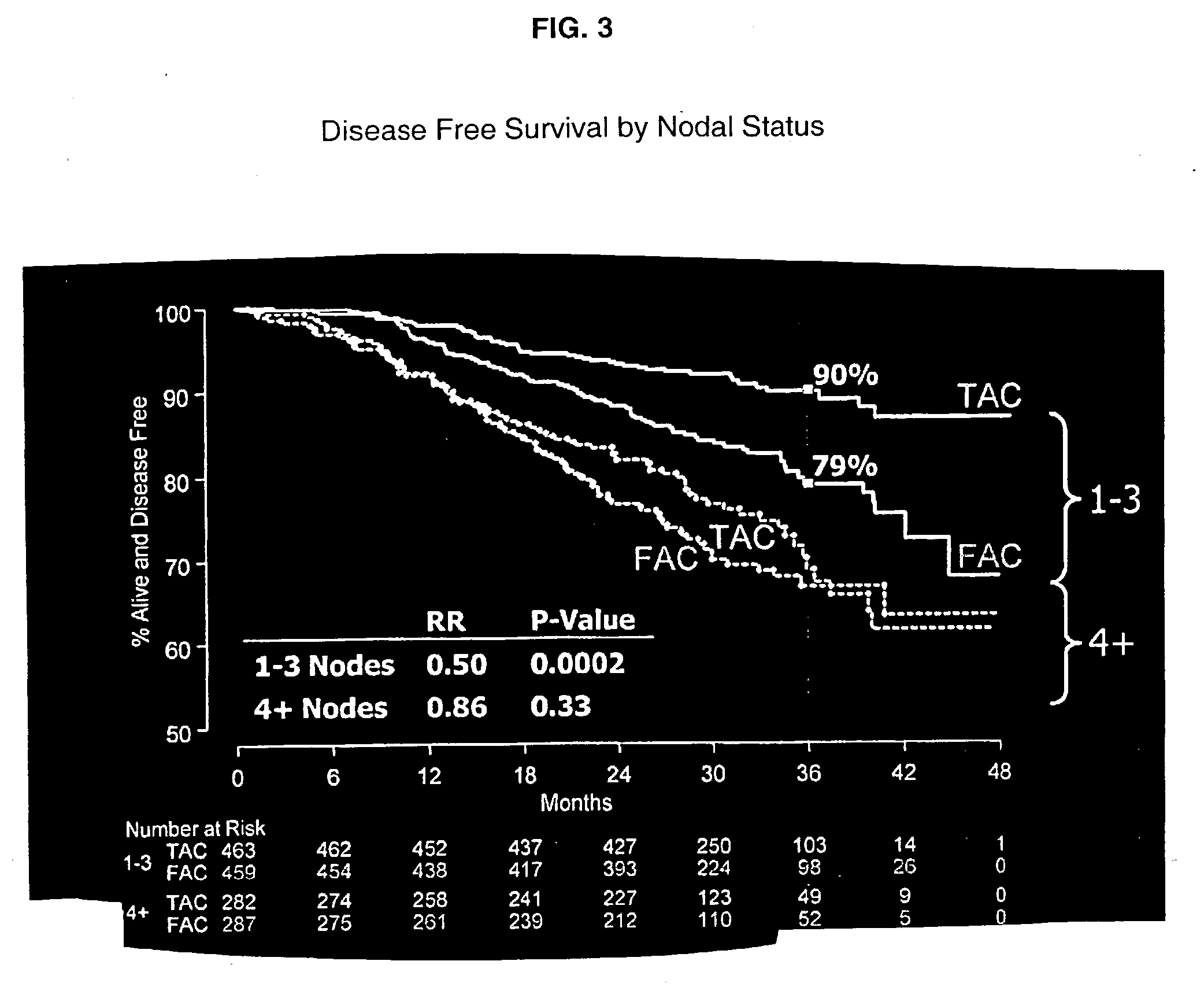
Use of docetaxel/doxorubicin/cyclophosphamide in adjuvant therapy
The present invention relates to a new method of adjuvant therapy in the treatment of early breast cancer, comprising administering six cycles of docetaxel, doxorubicin and cyclophosphamide to a patient in need thereof, wherein said dosages have a marked therapeutic effect when compared to other adjuvant therapies.
Owner:AVENTIS PHARMA SA (US)
Minimally invasive diagnosis and treatment for breast cancer
InactiveUS7769432B2Ultrasonic/sonic/infrasonic diagnosticsSurgical needlesCytologyEarly breast cancer
The present invention provides a treatment method to excise a cancerous lesion, such as in the breast, with subsequent ablation of the margin. The method provides for location and excision with ablation under open guidance or guided imaging and for diagnosis by cytology. The method may be a minimally, invasive same day method with diagnosis before or immediately after excision. Also provided is a method of treating close or positive margins of an excisional site of a cancerous lesion in a breast by ablating the margin while monitoring the fluorescence of a fluorophor at the site to determine when ablation of the close or positive margin has occurred.
Owner:INNOBLATIVE DESIGNS INC
Breast cancer detecting marker as well as detecting method, kit and biological chip thereof
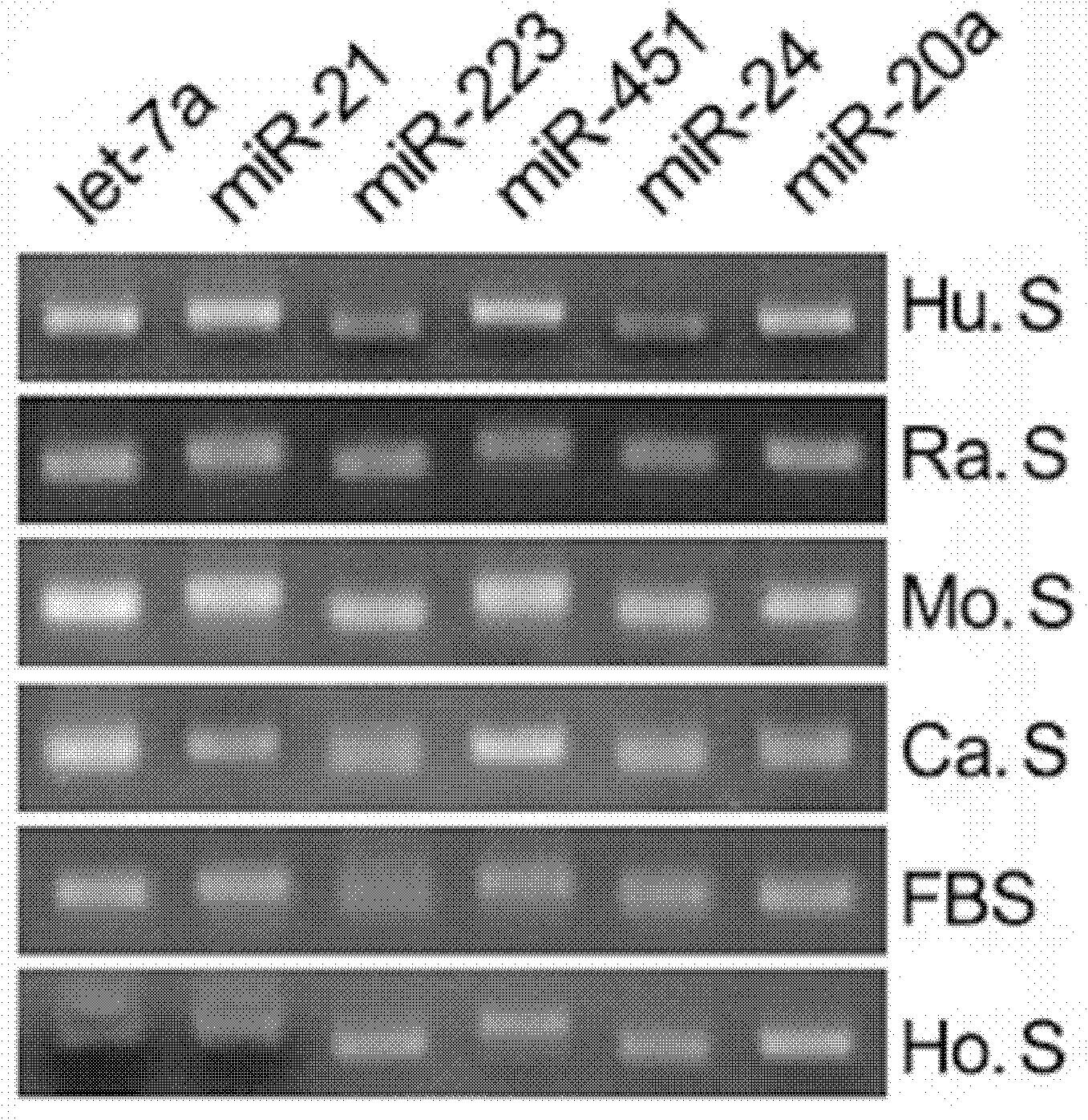
InactiveCN101988061AEasy to storeWide detection rangeMicrobiological testing/measurementDNA/RNA fragmentationHuman bodyEarly breast cancer
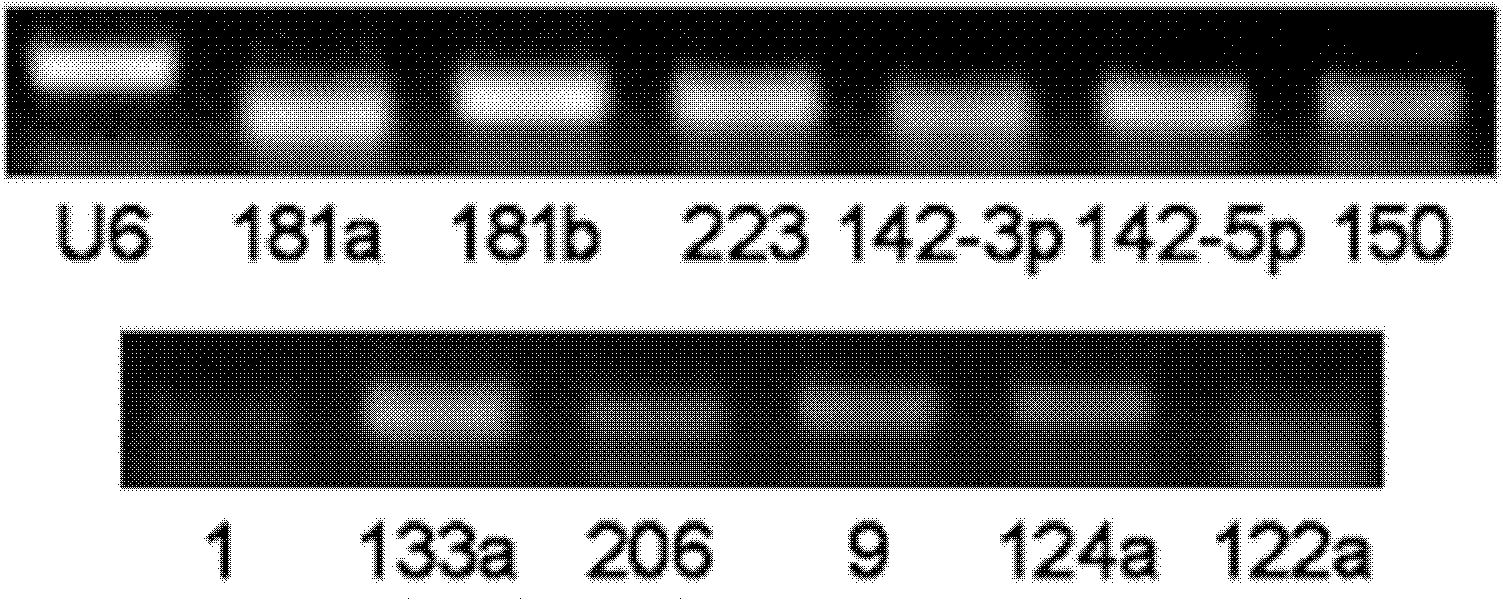
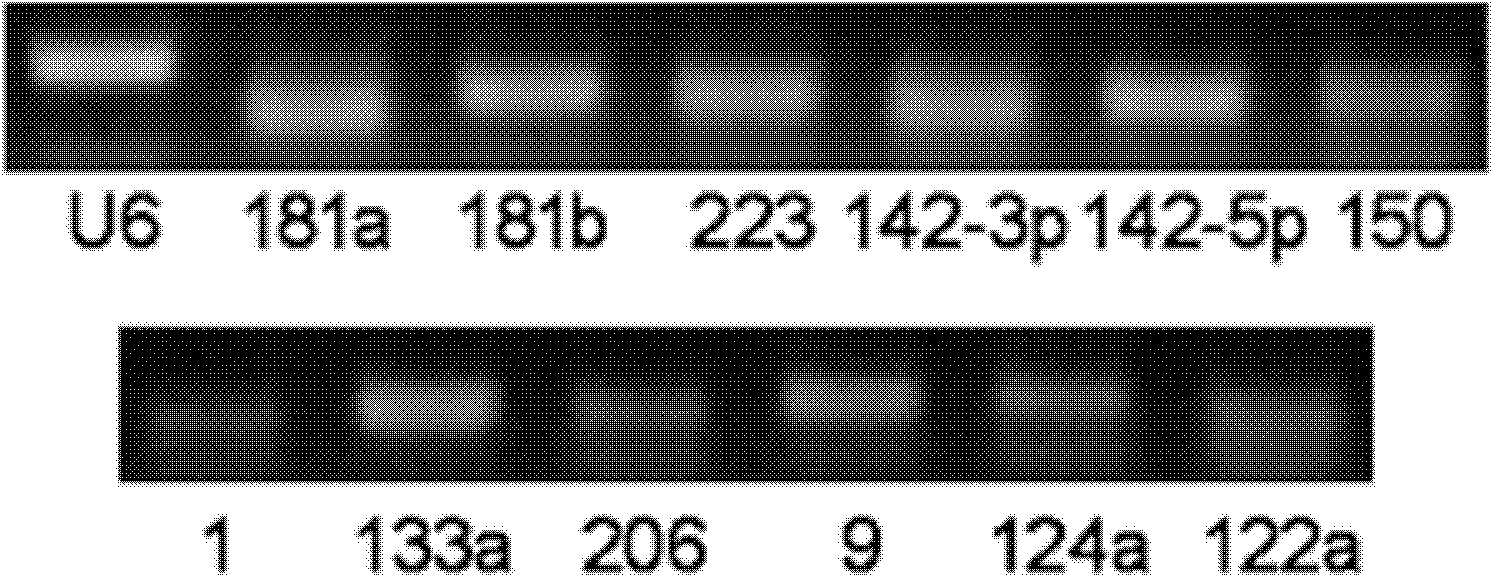
The invention relates to a breast cancer detecting marker by utilizing 16 specific tiny ribonucleic acids steadily existing in blood serum and blood plasma of a human body as well as a detecting method, a kit and a biological chip thereof, and the detecting marker can be used in the aspects of diagnosis and differential diagnosis of breast cancer, occurrence and recurrence forecast as well as efficacy assessment of disease complications, screening and therapeutic evaluation of medicinal active ingredients and the like, and has the advantages of wide detection pedigree, high sensitivity and low detection cost, the materials are conveniently taken, the samples are easy to store and the like. The method can be widely used in related work such as breast cancer census, the defects of low specificity and low sensitivity caused by insuperable individual difference of a single marker can be improved and the clinical detectable rate of the breast cancer is notably improved, and is an effective mean of early breast cancer diagnosis.
Owner:JIANGSU MICROMEDMARK BIOTECH
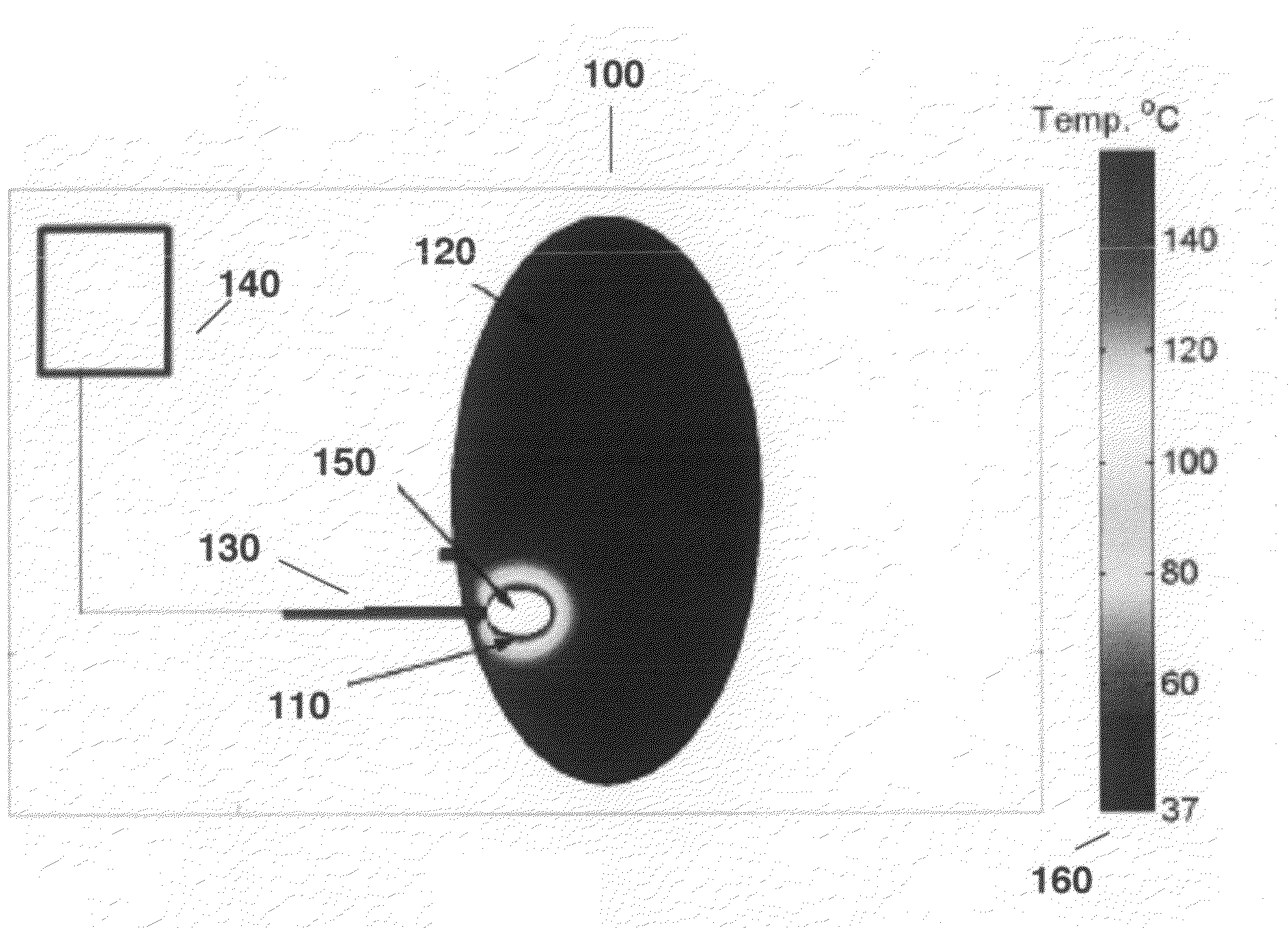
Intelligent bra for early detection of breast cancer
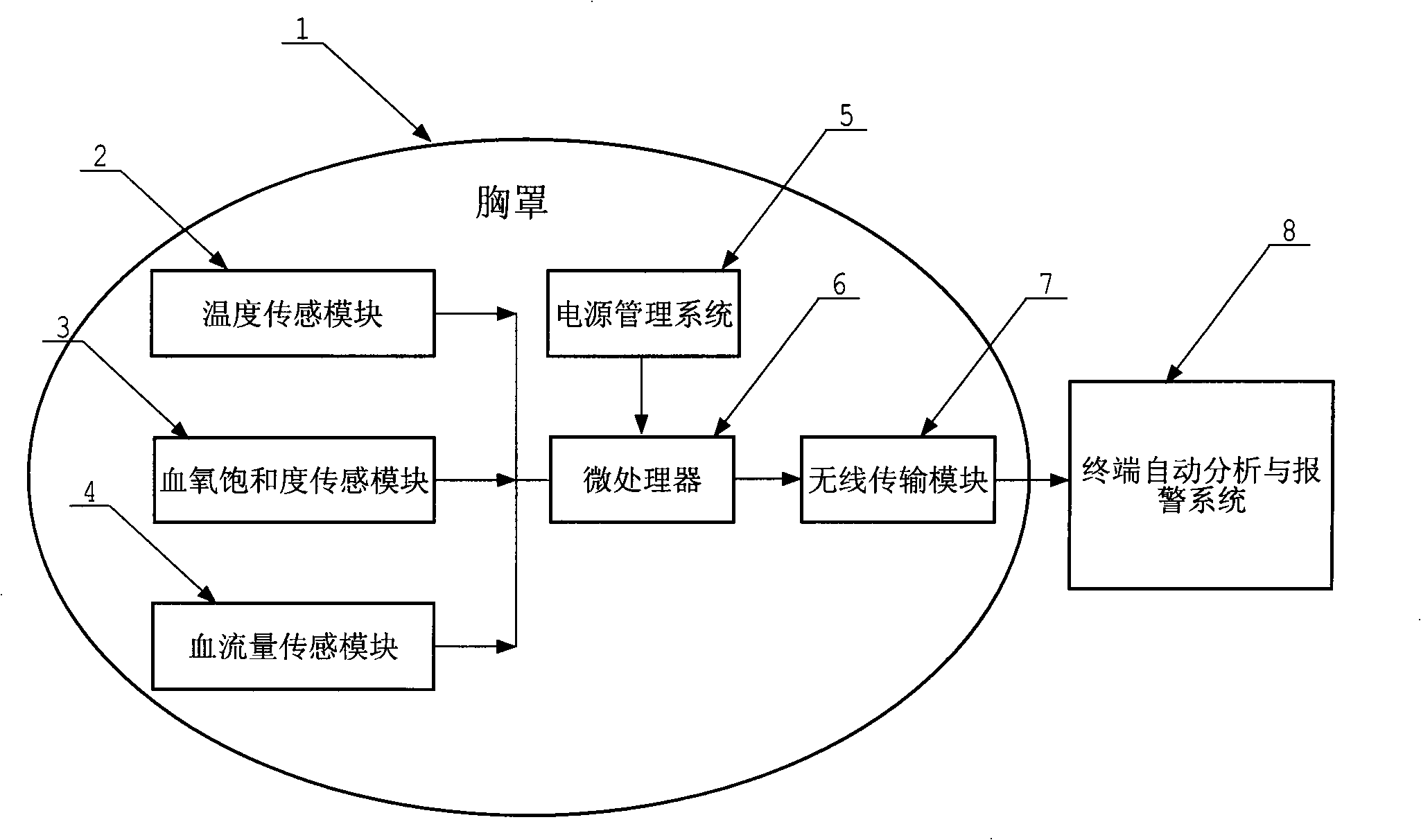
InactiveCN104223385AChange the prevention modelSave livesBrassieresDiagnostic recording/measuringWireless transmissionMedicine
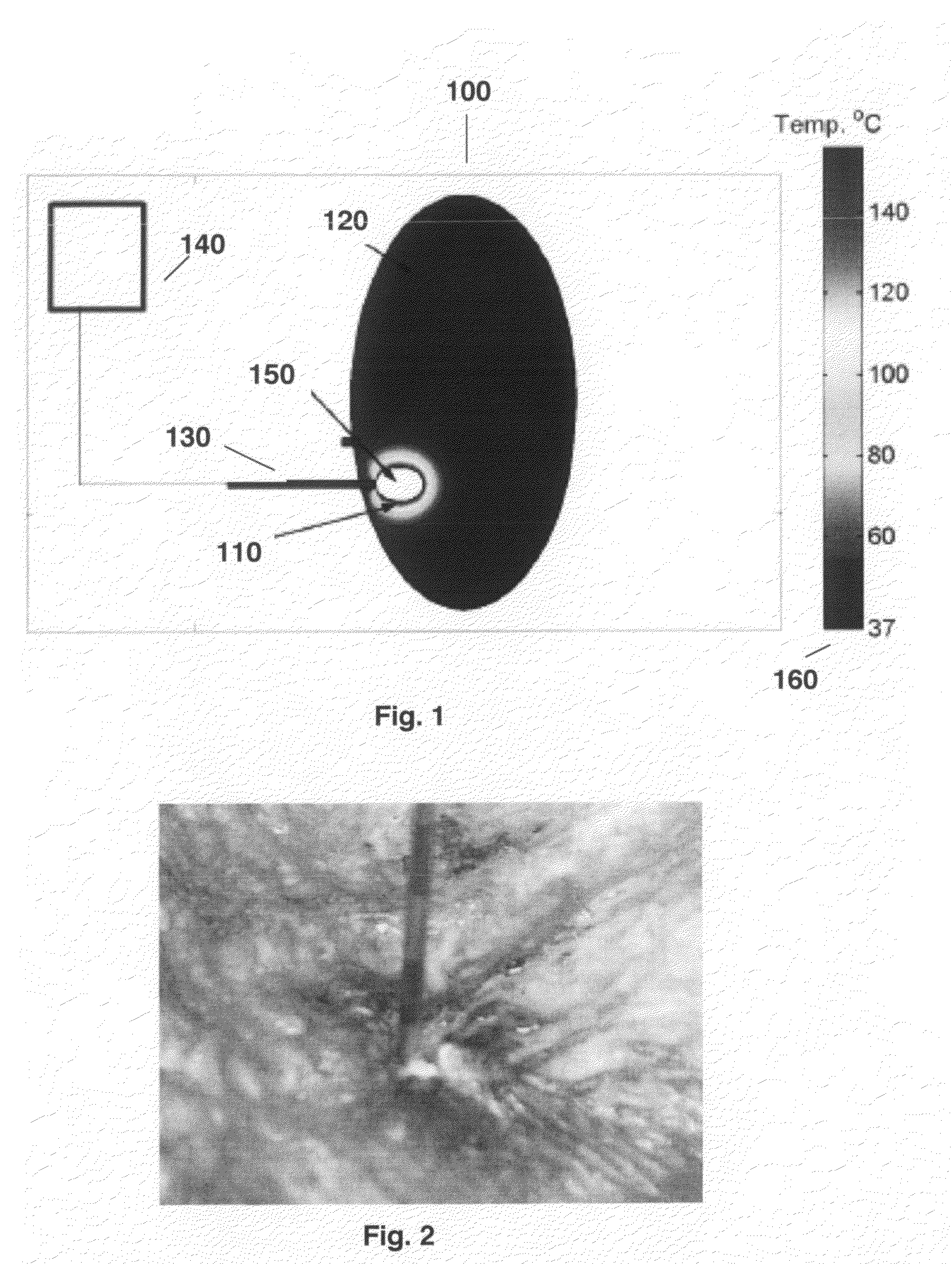
The invention relates to a novel intelligent bra for early detection of breast cancer. The intelligent bra carries ultra-high sensitive computer micro-processing chips, which include temperature sensor, blood flow sensors, oxyhemoglobin quantity sensors and the like for all-round acquisition of breast tissue temperature, blood flow and the hemoglobin amount. The device saves periodically collected data in a computer through a wireless transmission system, and automatically analyzes and alarms whether carcinogenesis possibility exists in breasts.
Owner:NANKAI UNIV
Apparatus and method for interstitial laser therapy of small breast cancers and adjunctive therapy
Apparatus and method for performing interstitial laser therapy and adjunctive therapy on a patient are revealed. The apparatus employs a combination of a mammography unit, an interstitial laser treatment device attached to the mammography unit and a treatment platform positioned relative to the mammography unit to enable the interstitial laser therapy to be performed. The use of the treatment platform with the mammography unit enables the interstitial laser therapy to be performed and, if necessary, adjunctive therapy to be performed in the same treatment room without transferring the patient to a new platform.
Owner:NOVIAN HEALTH INC
Image-guided removal and thermal therapy of breast cancer
InactiveUS20140025056A1Surgical navigation systemsSurgical instruments for heatingEarly breast cancerGeneral surgery
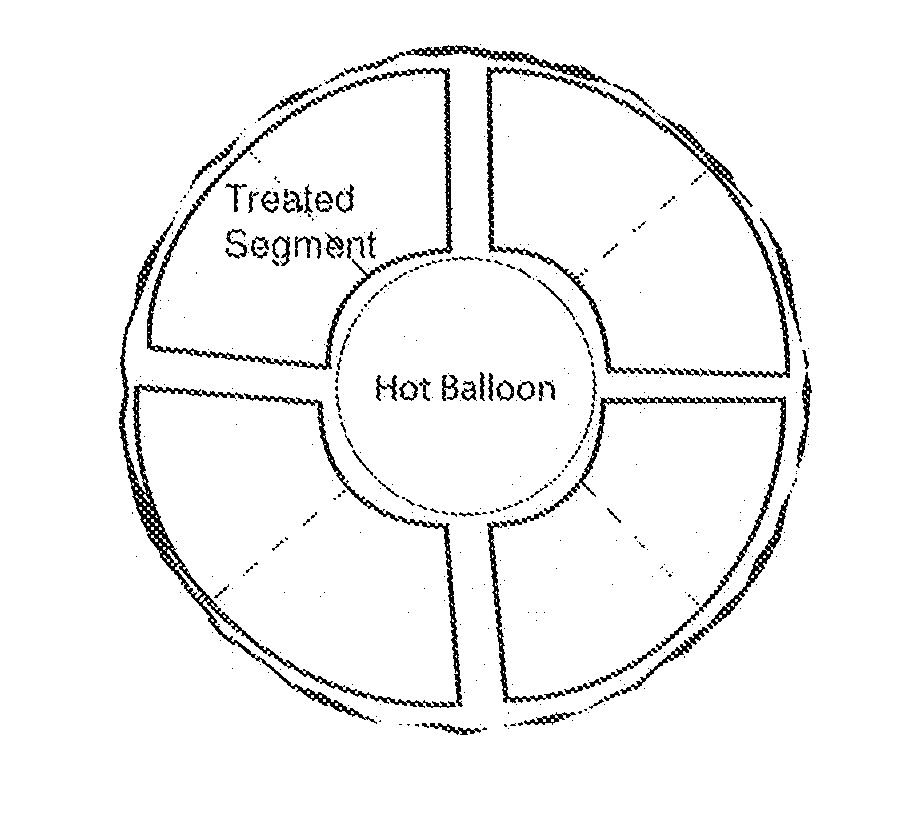
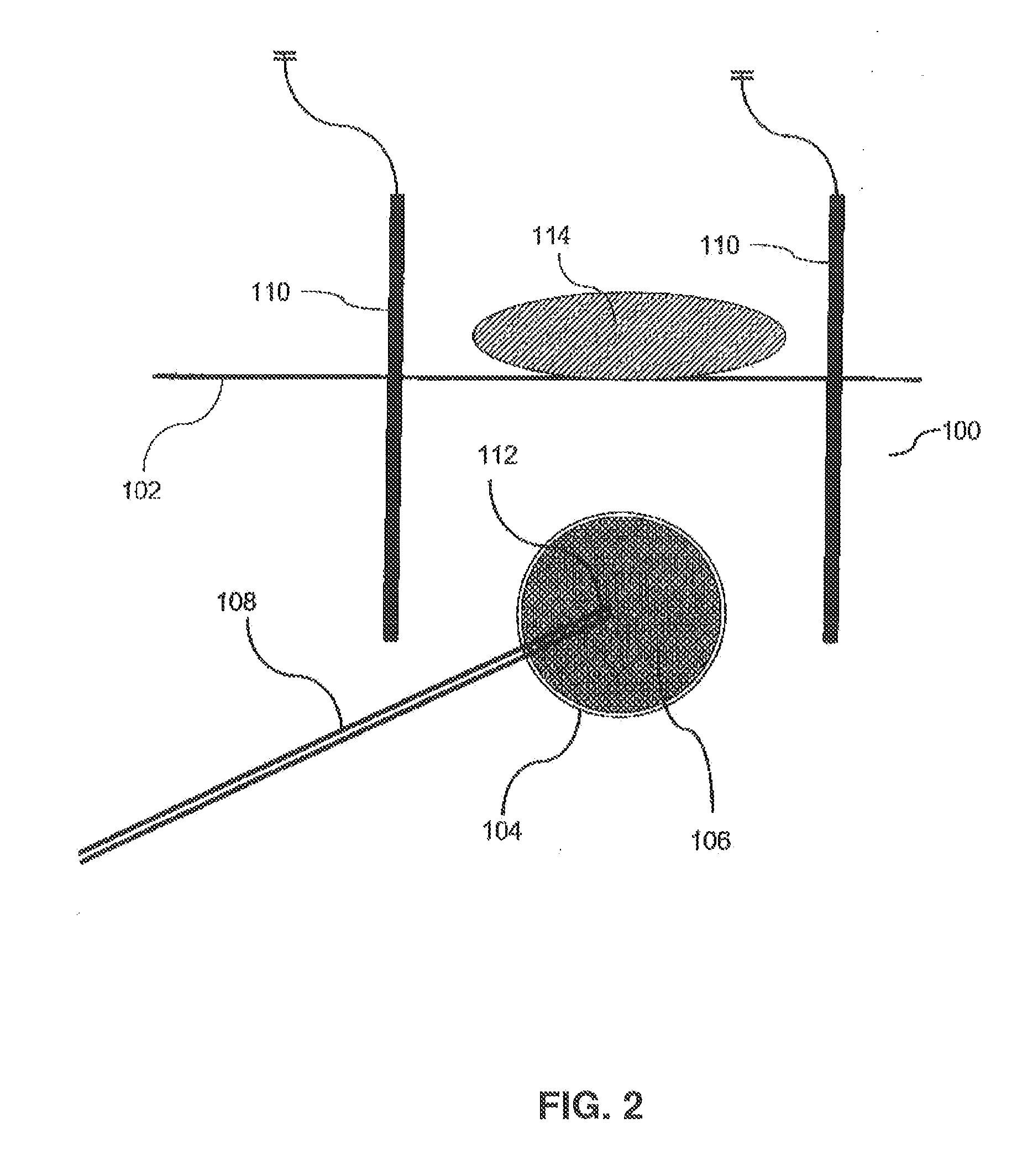
A method of removing undesirable cells and killing cells surrounding a cavity caused by the removal of the undesirable cells includes the step of inserting a probe through an incision made in the skin of a patient. The method further includes the steps of removing undesirable cells using the probe and using image-guided technology and inserting a balloon into the cavity formed by the removal of the undesirable cells. Still further, the method includes the steps of conforming the balloon to a shape of the cavity and providing sufficient fluid to fill the cavity based on the volume and reaching a temperature in the fluid to achieve a temperature in the tissue surrounding the cavity that kills cells surrounding the cavity.
Owner:DOWLATSHAHI KAMBIZ
Method and equipment for image-guided stereotactic radiosurgery of breast cancer
ActiveUS20100094119A1Monitor safetyMonitor operationMagnetic measurementsSterographic imagingStereotactic neurosurgeryEarly breast cancer
A method of treating a cancerous region in a breast of a patient comprising (i) imaging the breast in a three-dimensional coordinate system, (ii) stereotactically determining the location of the cancerous region in the breast, (iii) optionally determining the volume of the entire cancerous region to be treated, and (iv) while maintaining the breast in a three-dimensional coordinate system that is identical to or corresponds with the three-dimensional coordinate system used in (i), noninvasively exposing the cancerous region of the breast of the patient to a cancer-treatment effective dose of radiation; and equipment for use in such a method.
Owner:XCISION MEDICAL SYST +1
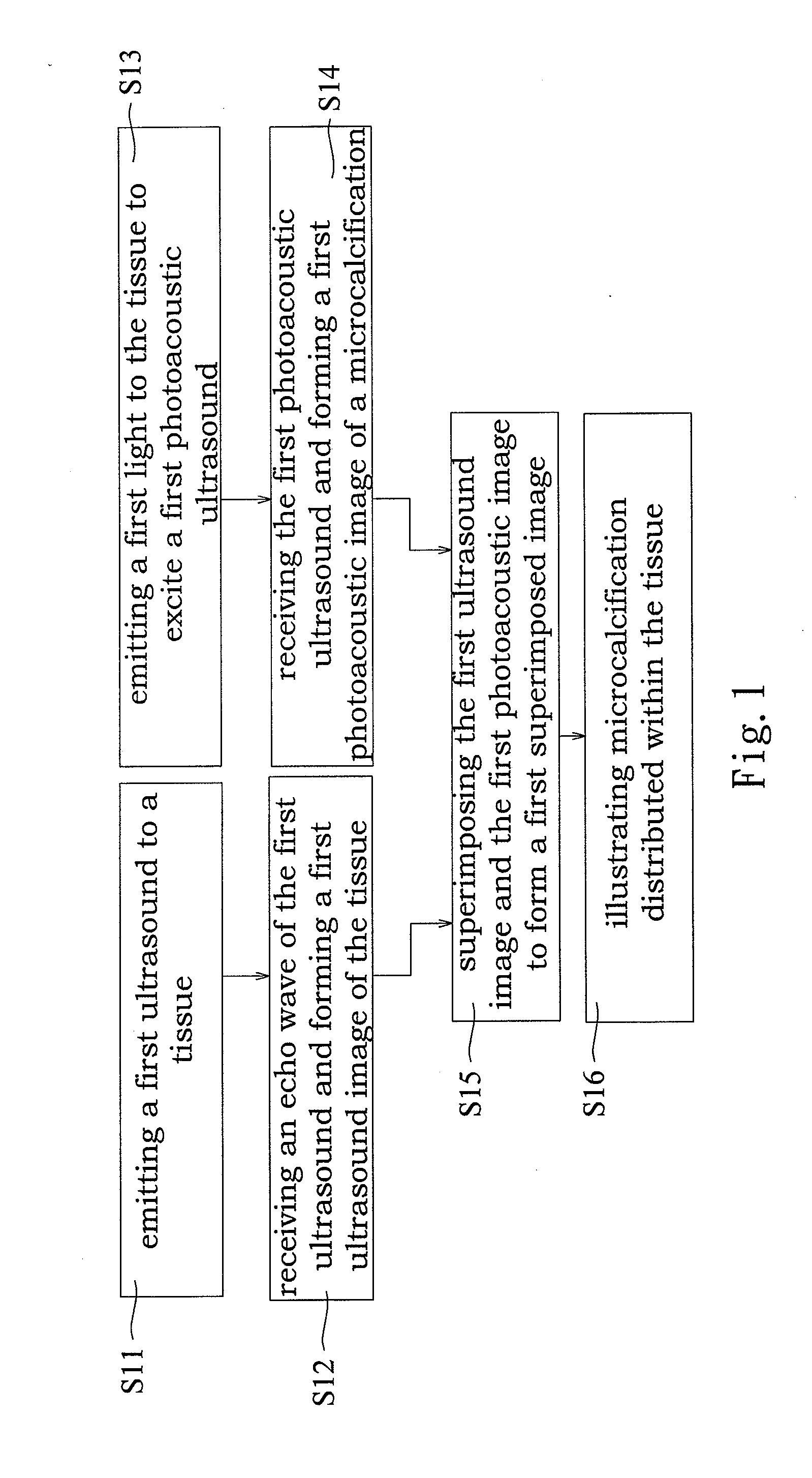
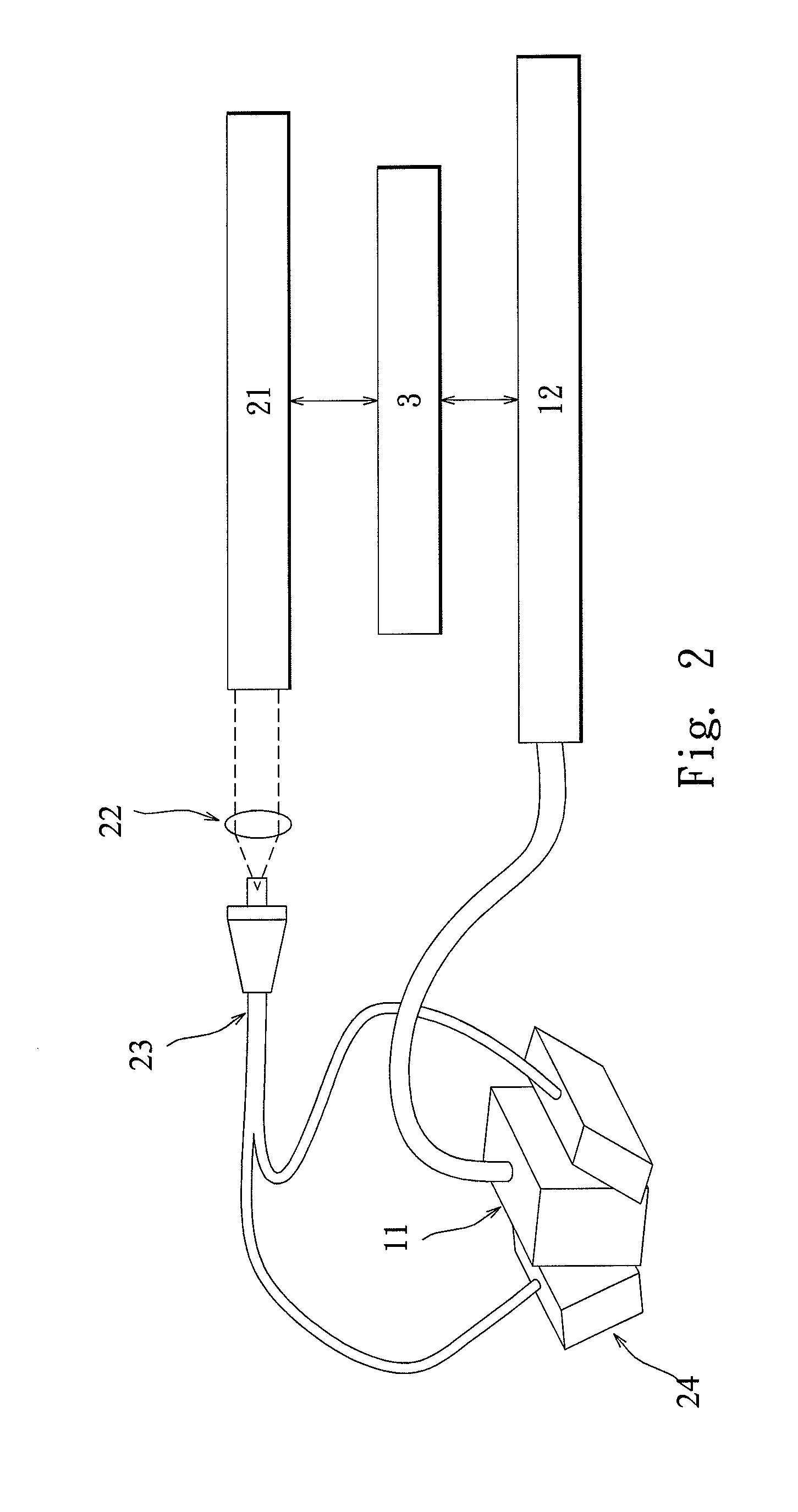
Imaging method for microcalcification in tissue and imaging method for diagnosing breast cancer
InactiveUS20110144496A1High optical contrastHigh resolutionBlood flow measurement devicesInfrasonic diagnosticsImage resolutionEarly breast cancer
An imaging method for microcalcification displays microcalcification distribution by acquiring and overlapping a photoacoustic image of microcalcification and an ultrasonic image of tissue. The image acquired by the present invention, in comparison to images acquired by ultrasonic and X-ray mammography, has advantages in no speckle noises, higher optical contrast, higher ultrasonic resolution, and so on. The present invention also has advantage in safety by adopting a light source having no ionizing radiation. An imaging method for diagnosing breast cancer is also herein disclosed.
Owner:NATIONAL TSING HUA UNIVERSITY
Immunodiagnosis reagent kit for breast cancer and testing sheet
The disclosed testing block for Her2 express in mammary tissue includes: the Her2 high-express area, the express contrast area, the no-express contrast area, and the testing area for target tissue. This invention can provide accurate evaluation standard for clinic diagnosis on breast cancer.
Owner:SHANGHAI ZHANGJIANG BIOTECH
Gastric cancer detection marker and detecting method thereof, kit and biochip
ActiveCN101988059AEasy to storeWide detection rangeMicrobiological testing/measurementDNA/RNA fragmentationAdditive ingredientEarly breast cancer
The invention relates to a gastric cancer detection marker, and a detecting method thereof, a related kit and a biochip. The gastric cancer detection marker comprises 21 specific micro RNAs stable in human serum / plasma. The marker which is broad in detection spectrum, high in sensitivity, low in detection cost, easy to be got and stored can be used for diagnosing and differentially diagnosing the gastric cancer, predicating occurrence and reoccurrence of diseases and conditions, evaluating therapeutic efficiency and efficacy and screening active pharmaceutical ingredients and the like. The method can be widely applied to cancer survey and related work, improves low specificity and low sensitivity caused by individual difference which is difficult for a single marker to overcome, and remarkably improves the clinical detection rate of the gastric cancer. Therefore, the method is an effective means to diagnose early breast cancer.
Owner:JIANGSU MICROMEDMARK BIOTECH
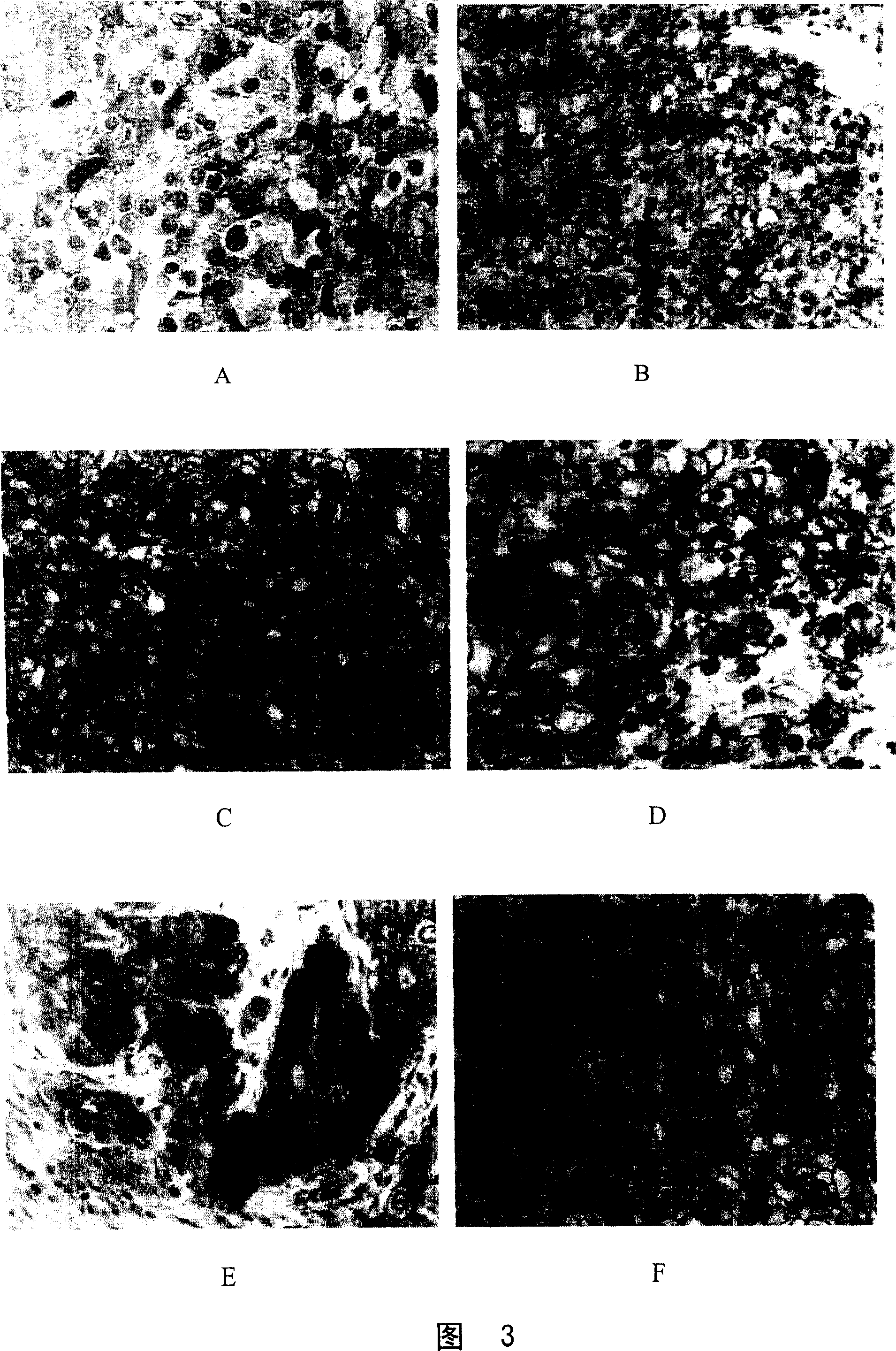
Processing method and device for pathological image of lymphatic metastasis of breast cancer
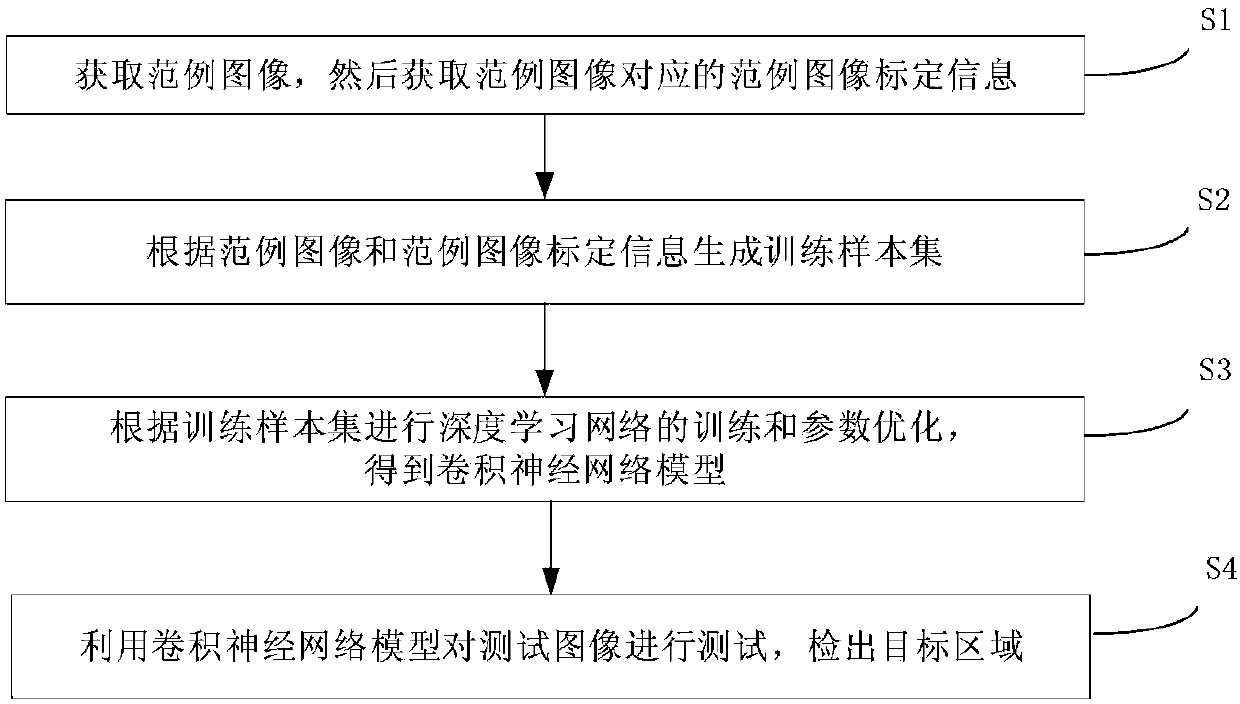
InactiveCN107945181AImprove work efficiencyReduce error rateImage analysisImage calibrationEarly breast cancer
The invention provides a processing method and device for a pathological image of lymphatic metastasis of a breast cancer. The method comprises: S1, an example image is obtained and example image calibration information corresponding to the example image is obtained; S2, according to the example image and the example image calibration information, a training sample set is generated; S3, deep learning network training and parameter optimization are carried out based on the training sample set to obtain a convolutional neural network model; and S4, a testing image is tested by using the convolutional neural network model and a target area is detected. According to the processing method and device, automatic image classification is realized by using the deep learning technology; technical problems of poor consistency and low accuracy of the manual film reading and classification work are solved; the work efficiency is improved; and the probability of errors is reduced.
Owner:北京羽医甘蓝信息技术有限公司
Method for detecting cancer
The invention concerns methods and compositions that can be used for detecting cancer in mammals, particularly humans. The invention particularly concerns serum markers of cancers and their use in diagnostic procedures. The invention also concerns tools and / or kits that can be used for carrying out these methods (reagents, probes, primers, antibodies, chips, cells, etc., the preparation thereof and their use. The invention can be used for detecting the presence or the progression of a cancer in mammals, particularly breast cancer including during early stages.
Owner:BIOMERIEUX SA +1
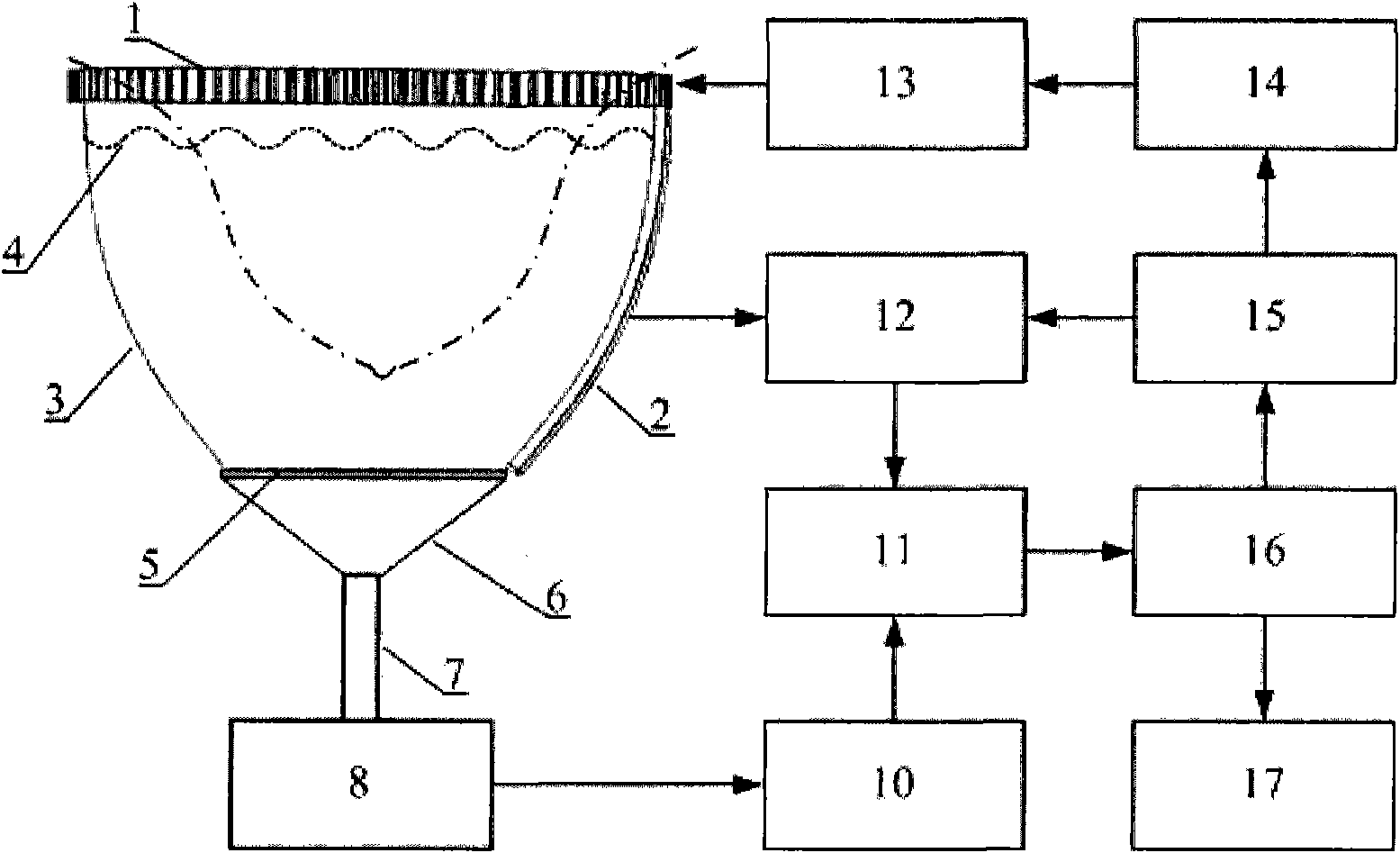
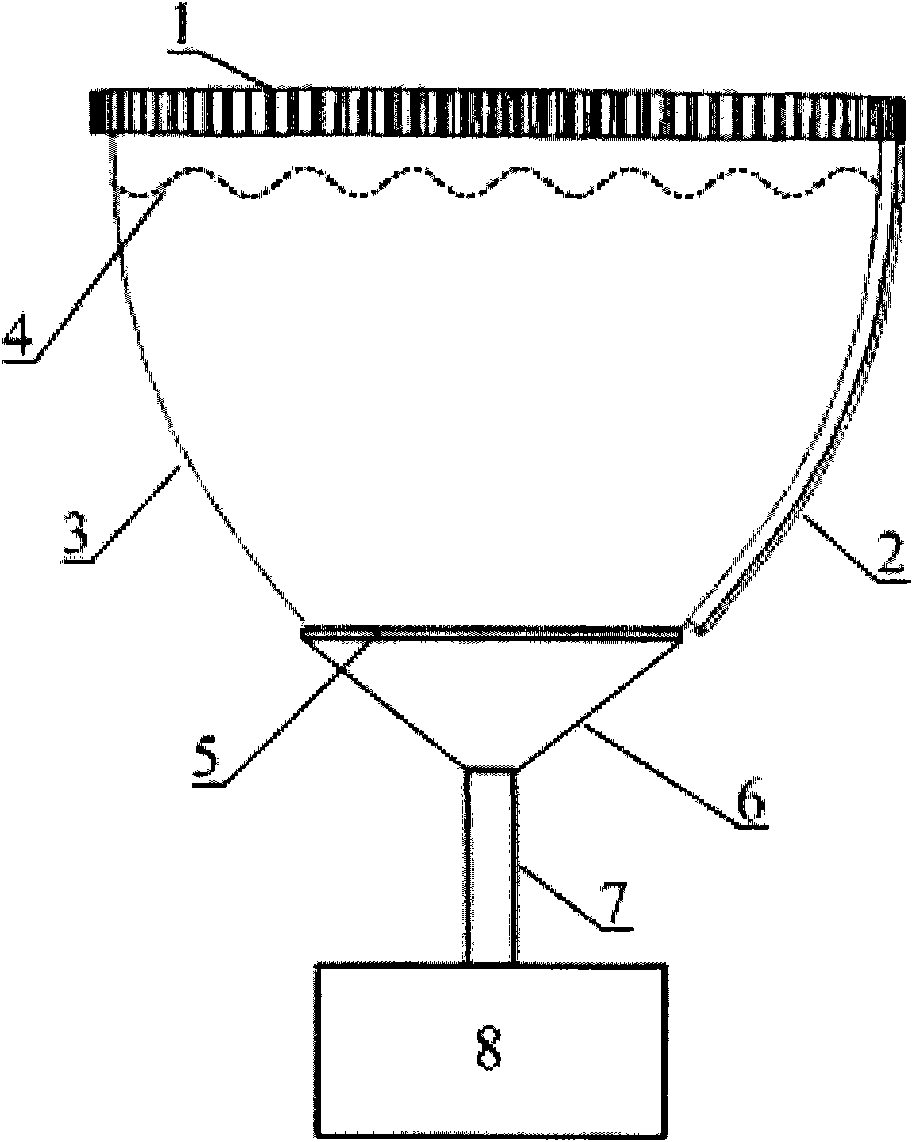
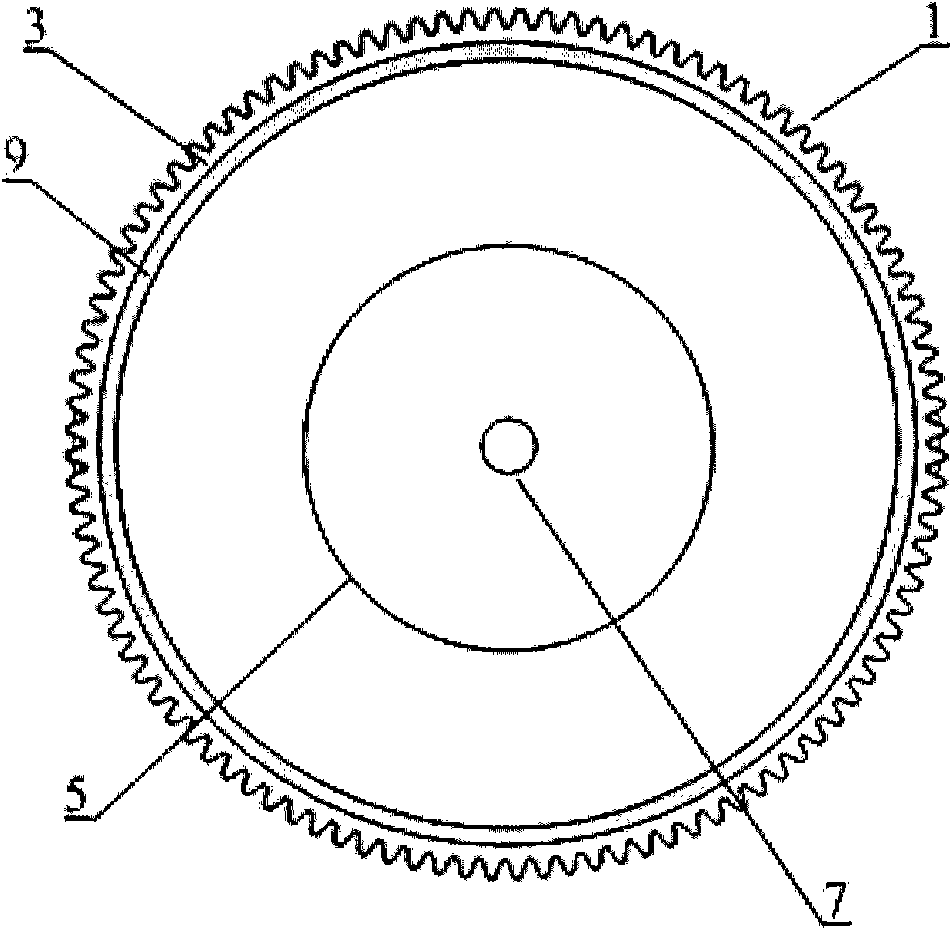
Early-stage breast cancer nondestructive screening and imaging system
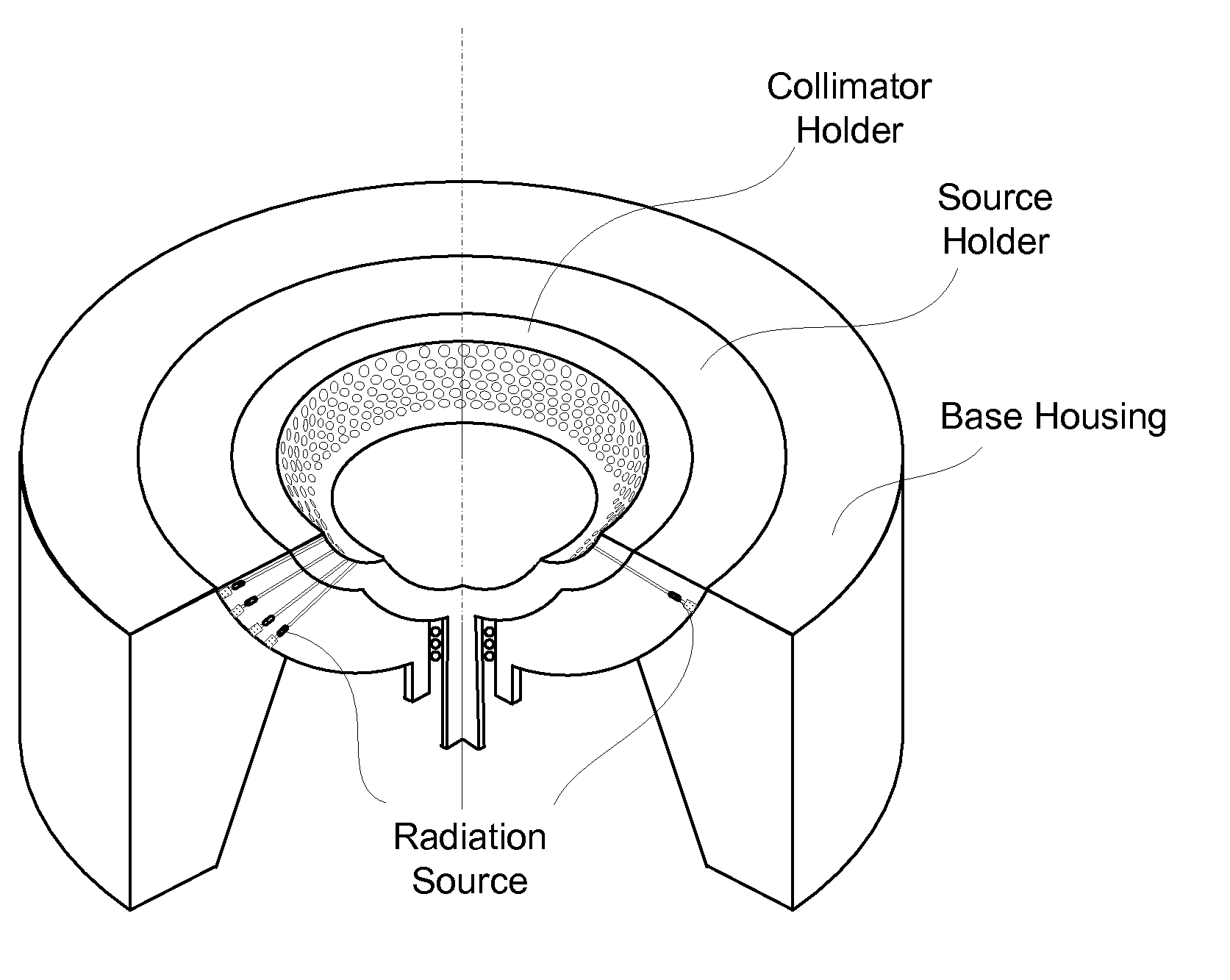
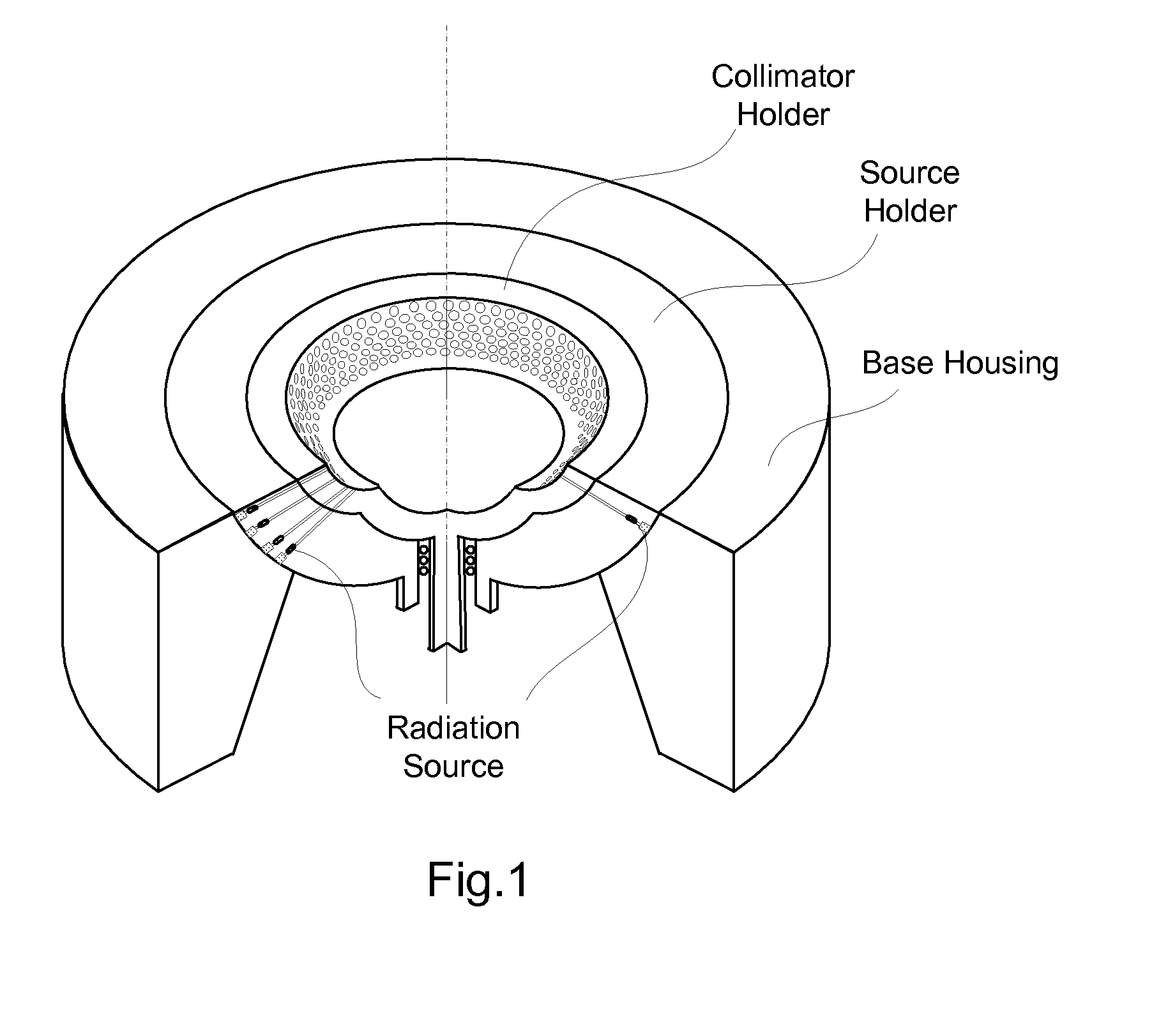
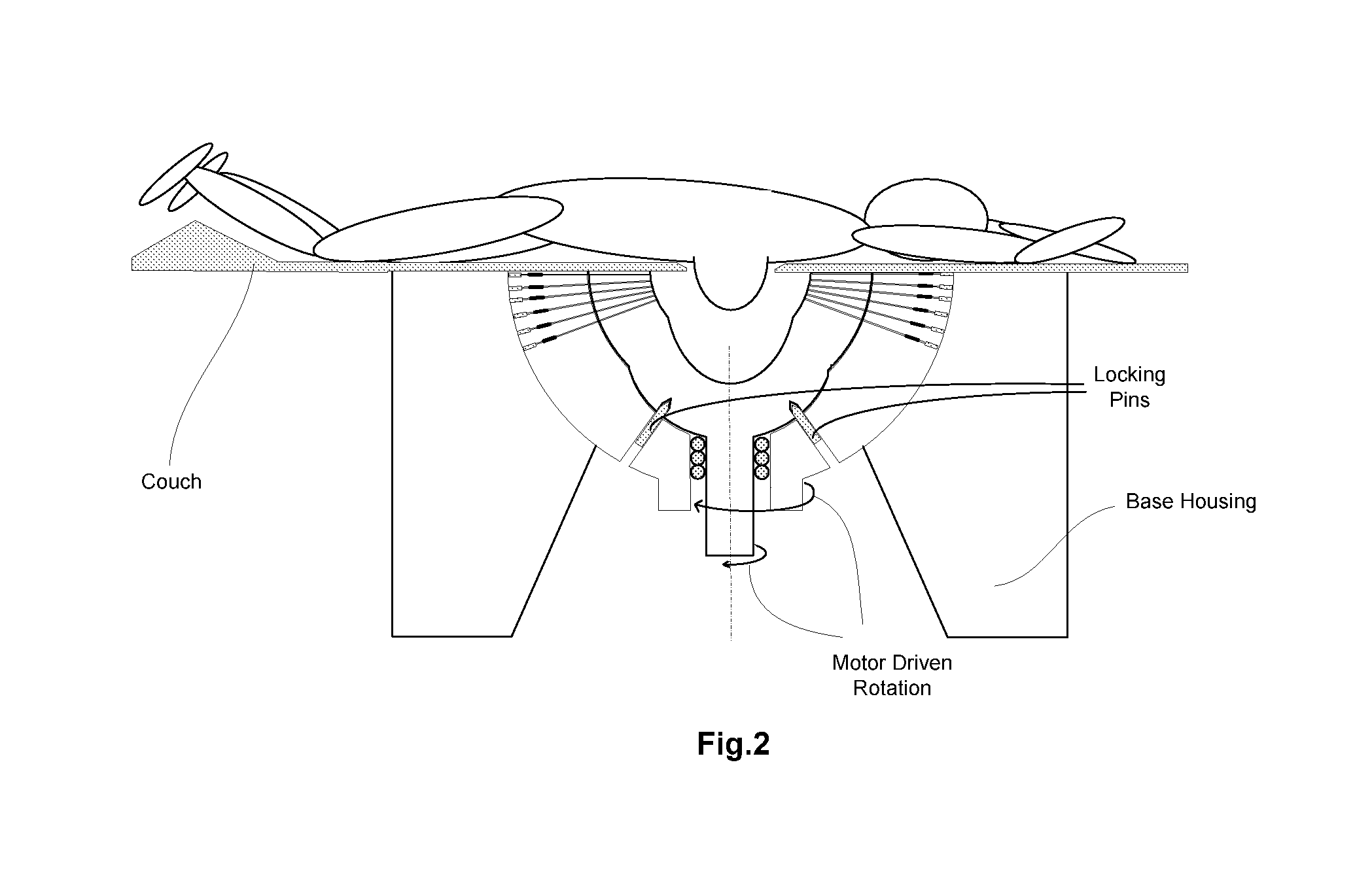
InactiveCN101816572AMiniaturizationImprove practicalityUltrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsEarly breast cancerPulse microwave
The invention discloses an early-stage breast cancer nondestructive screening and imaging system, which mainly comprises circular gears, an annular ultrasonic array, a bowl-shaped annular shell, ultrasonic coupling liquid, a protective film, a horn antenna, a waveguide tube, a microwave generator, a frequency divider, a data acquisition circuit, a preprocessing circuit, a stepping motor, a driver, a digital I / O card, a computer and a display. The working process of the system is that: detected mammary glands are radiated by pulse microwaves to generate thermo-acoustic signals which are received by the annular ultrasonic array and are finally acquired by the computer; the driver drives the annular ultrasonic array to rotate to a next position around detected biological tissues; and the steps of acquisition and rotation are repeated until the thermo-acoustic signals of enough positions are received, and the computer re-establishes three-dimensional thermo-acoustic images of the detectedtissues. The early-stage breast cancer nondestructive screening and imaging system can quickly and nondestructively realize the three-dimensional thermo-acoustic imaging of the detected mammary glands to provide one of important bases for the diagnosis of early-stage breast cancer.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Molecular markers predicting response to adjuvant therapy, or disease progression, in breast cancer
Predicting response to adjuvant therapy or predicting disease progression in breast cancer is realized by (1) first obtaining a breast cancer test sample from a subject; (2) second obtaining clinicopathological data from said breast cancer test sample; (3) analyzing the obtained breast cancer test sample for presence or amount of (a) one or more molecular markers of hormone receptor status, one or more growth factor receptor markers, (b) one or more tumor suppression / apoptosis molecular markers; and (c) one or more additional molecular markers both proteomic and non-proteomic that are indicative of breast cancer disease processes; and then (4) correlating (a) the presence or amount of said molecular markers and, with (b) clinicopathological data from said tissue sample other than the molecular markers of breast cancer disease processes. A kit of (1) a panel of antibodies; (2) one or more gene amplification assays; (3) first reagents to assist said antibodies with binding to tumor samples; (4) second reagents to assist in determining gene amplification; permits, when applied to a breast cancer patient's tumor tissue sample, (A) permits observation, and determination, of a numerical level of expression of each individual antibody, and gene amplification; whereupon (B) a computer algorithm, residing on a computer can calculate a prediction of treatment outcome for a specific treatment for breast cancer, or future risk of breast cancer progression.
Owner:LINKE STEVEN +2
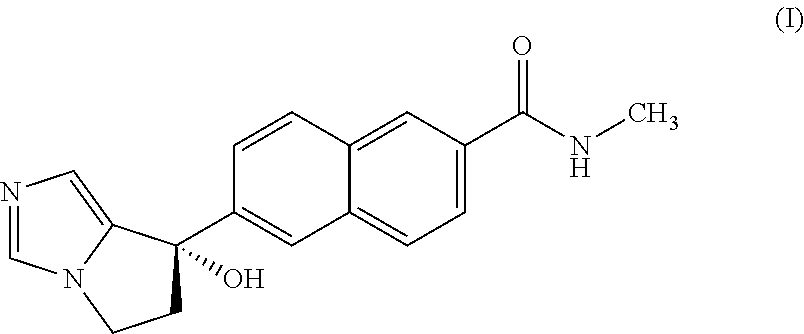
Methods of treating early breast cancer with trastuzumab-mcc-dm1 and pertuzumab
InactiveUS20170035907A1Improve responseExtension of timeOrganic active ingredientsAntibody ingredientsGynecologyEarly breast cancer
Methods of treating patients having HER2-positive, operable, locally advanced or inflammatory breast cancer with the antibody-drug conjugate Trastuzumab-MCC-DM1 and Pertuzumab are provided.
Owner:GENENTECH INC
Kit for evaluating prognostic risks of breast cancer
ActiveCN105200043AMeet testing needsEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationValidation testIndividualized treatment
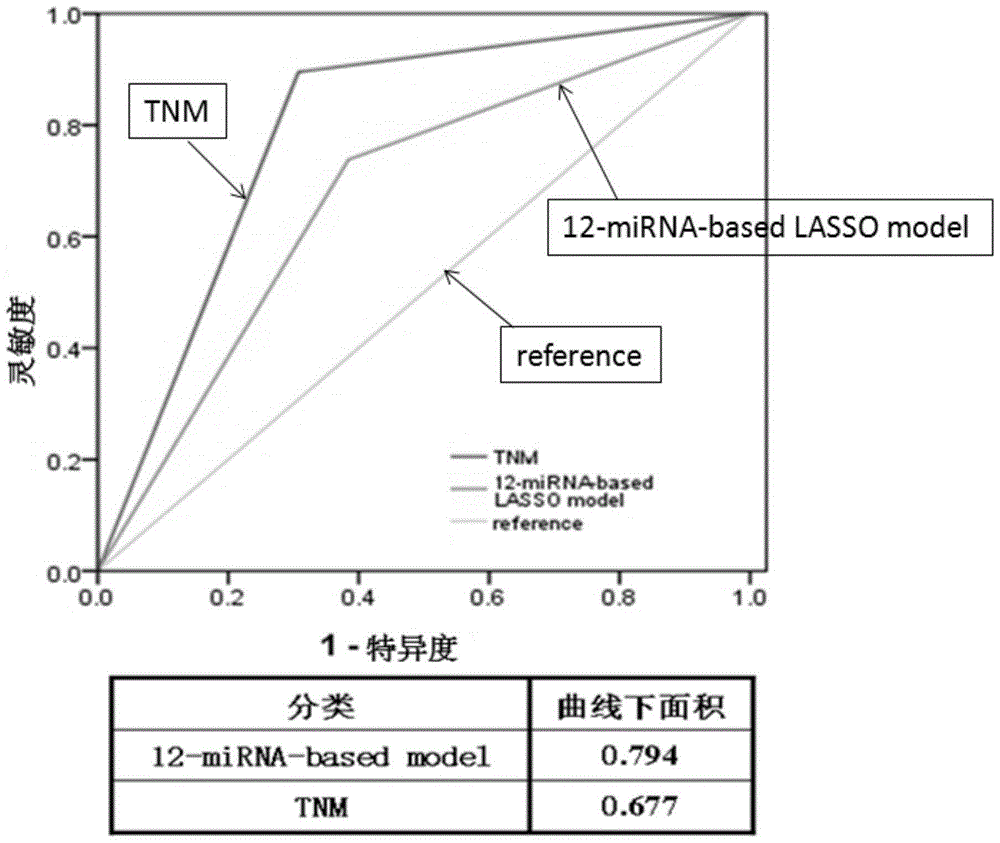
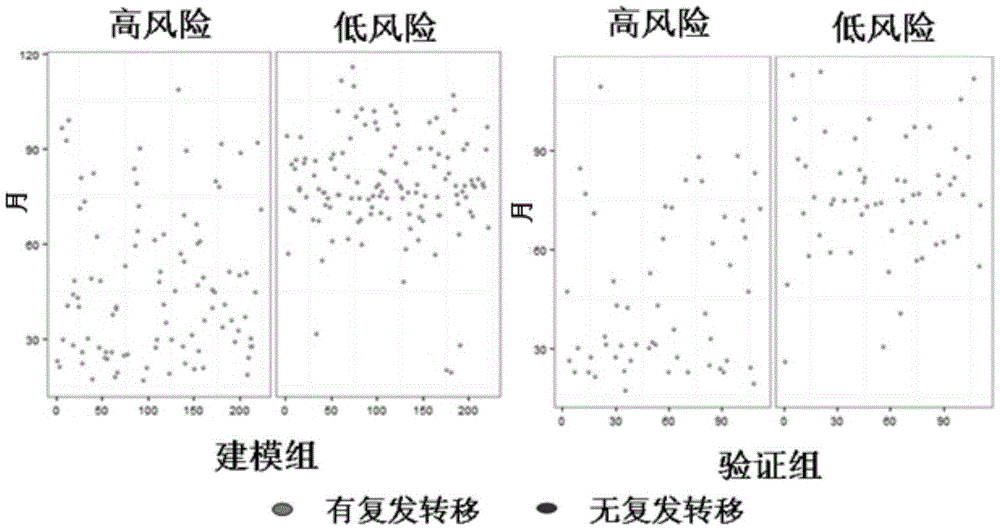
The invention provides a kit for evaluating prognostic risks of breast cancer. Big-data clinical validation test results show that 12 miRNAs are combined to be used as a molecular marker, and the molecular marker has an outstanding advantage on the aspect of evaluation of prognostic risks of ER / PR(+)HER2(-) breast cancer. The molecular marker formed by combining the 12 miRNAs is firstly applied to development of the miRNA detection kit of ER / PR(+)HER2(-) breast cancer. The breast cancer miRNA detection kit can realize prognosis evaluation of ER / PR(+)HER2(-) breast cancer on the basis of a real-time fluorogenic quantitative PCR method, and can screen patients with high relapse risks, guide clinical individualized treatment and improve treatment accuracy.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Method for examining prognosis of breast cancer
Disclosed is a method for examining prognosis of breast cancer including the steps of: (A) extracting RNA from a specimen collected from a subject, (B) preparing a determination sample using the extracted RNA, (C) determining the expression level of each gene in the specific gene groups using the obtained determination sample, (D) analyzing the expression level of the determined each gene, and (E) examining prognosis of breast cancer, based on the obtained analysis result are performed.
Owner:OSAKA UNIV +1
Apparatus and method for the treatment of breast cancer with particle beams
InactiveUS20100140500A1Reduced pressure environmentReduce pressureUltrasonic/sonic/infrasonic diagnosticsMaterial analysis by optical meansMedicineParticle beam
An apparatus and method for treating a tumor mass location in a breast of a patient. The breast is stabilized in space, preferably by creating a reduced pressure environment around the breast. Particle beams are applied to a tumor mass location positioned at the center of a substantially spherical (or cylindrical) portion of a chamber filled with a fluid having an electron density substantially similar to the breast. The patient treatment platform is translated to aim the particle beam at the tumor mass location while traversing and beaming perpendicular to the portion of the chamber.
Owner:PROCURE TREATMENT CENTS
Adjuvant treatment of her2-positive breast cancer
ActiveUS20180250397A1Reduce riskReduce deathOrganic active ingredientsPharmaceutical containersPrimary breast cancerEarly breast cancer
Methods are provided for the adjuvant treatment of operable HER2-positive primary breast cancer in human patients by administration of pertuzumab in addition to chemotherapy and trastuzumab. The methods reduce the risk of recurrence of invasive breast cancer or death for a patient diagnosed with HER2-positive early breast cancer (eBC) compared to administration of trastuzumab and chemotherapy, without pertuzumab.
Owner:F HOFFMANN LA ROCHE & CO AG
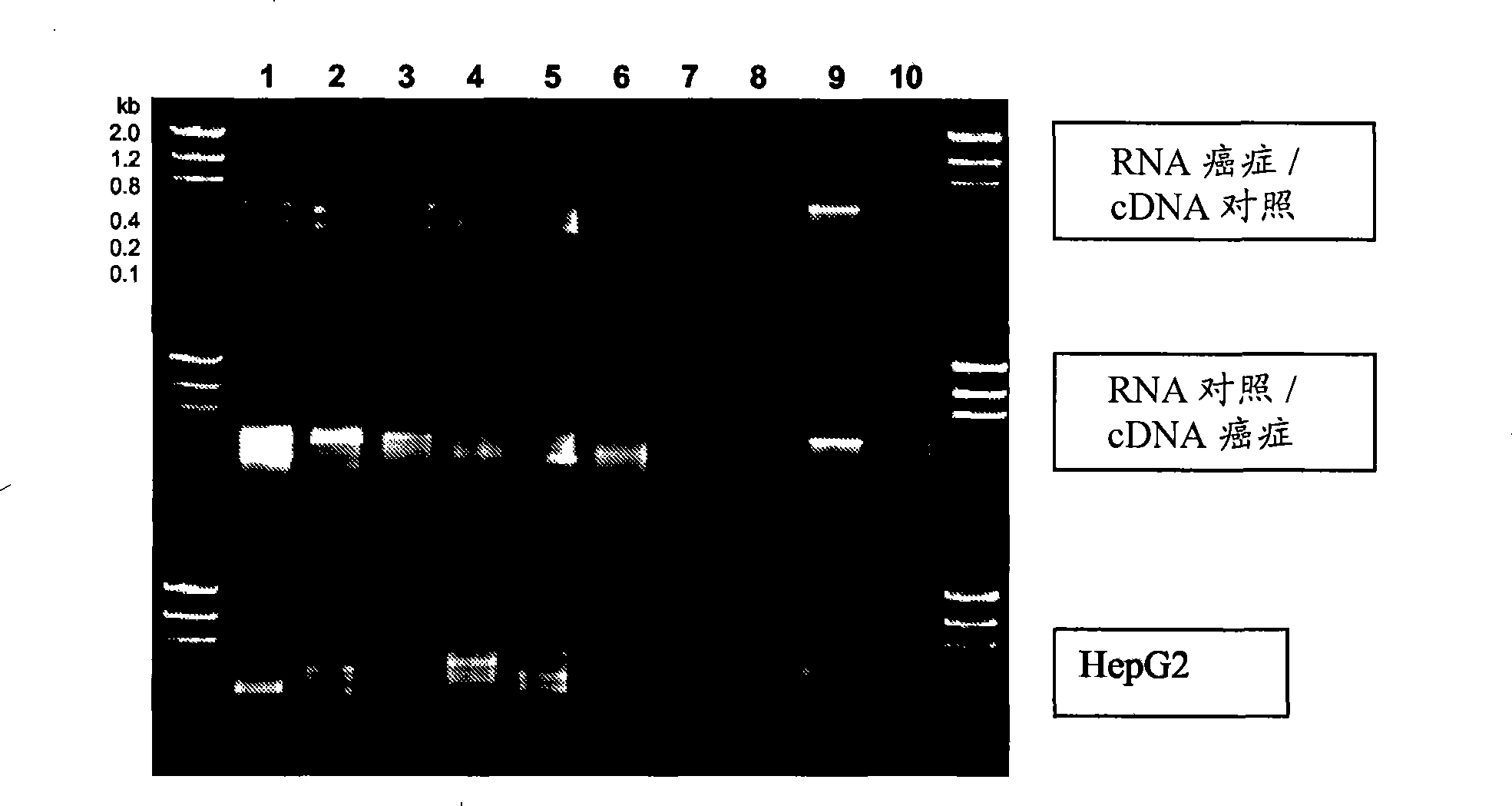
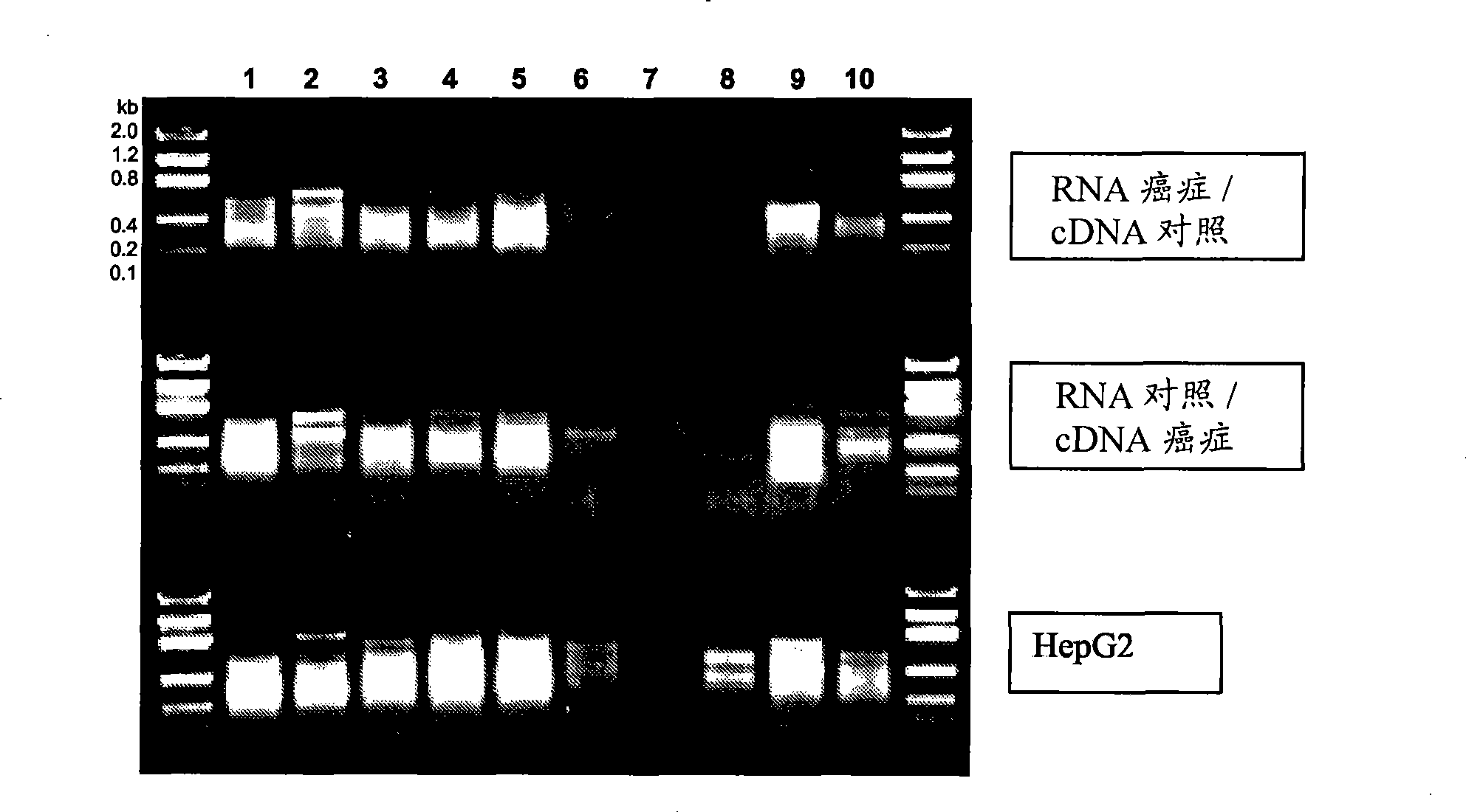
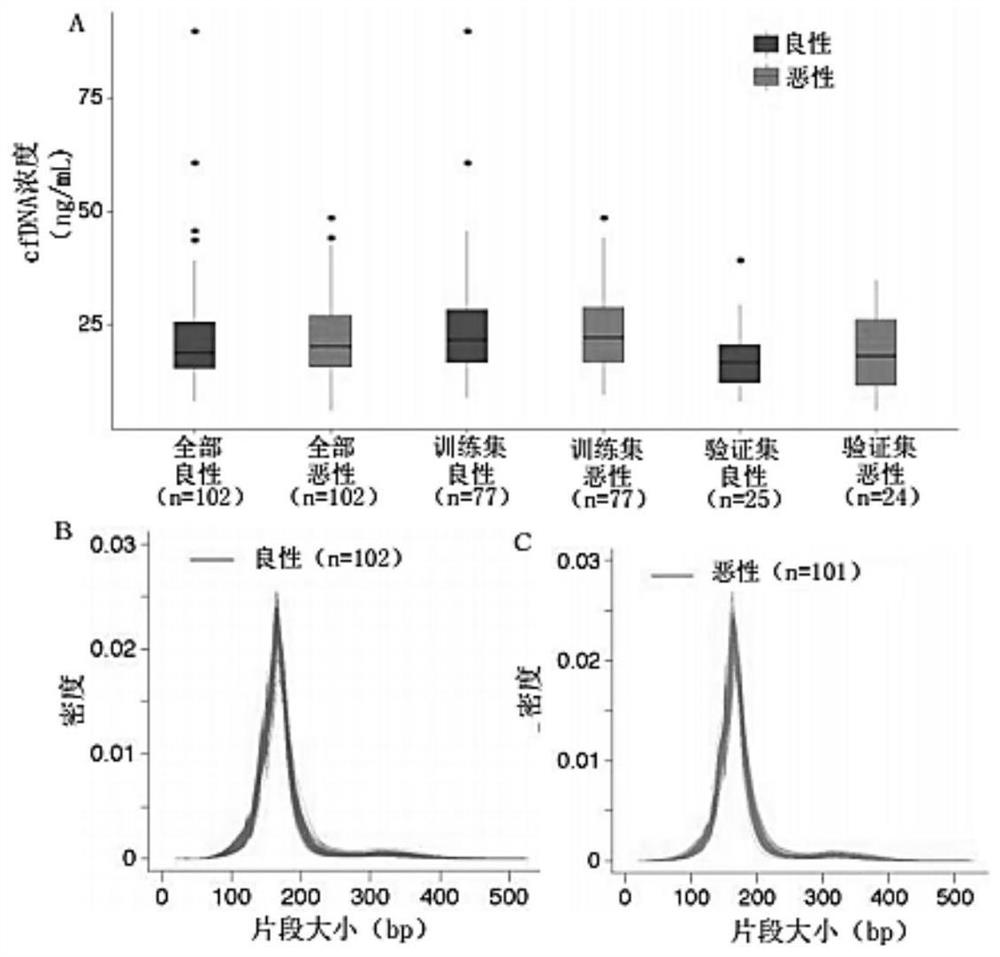
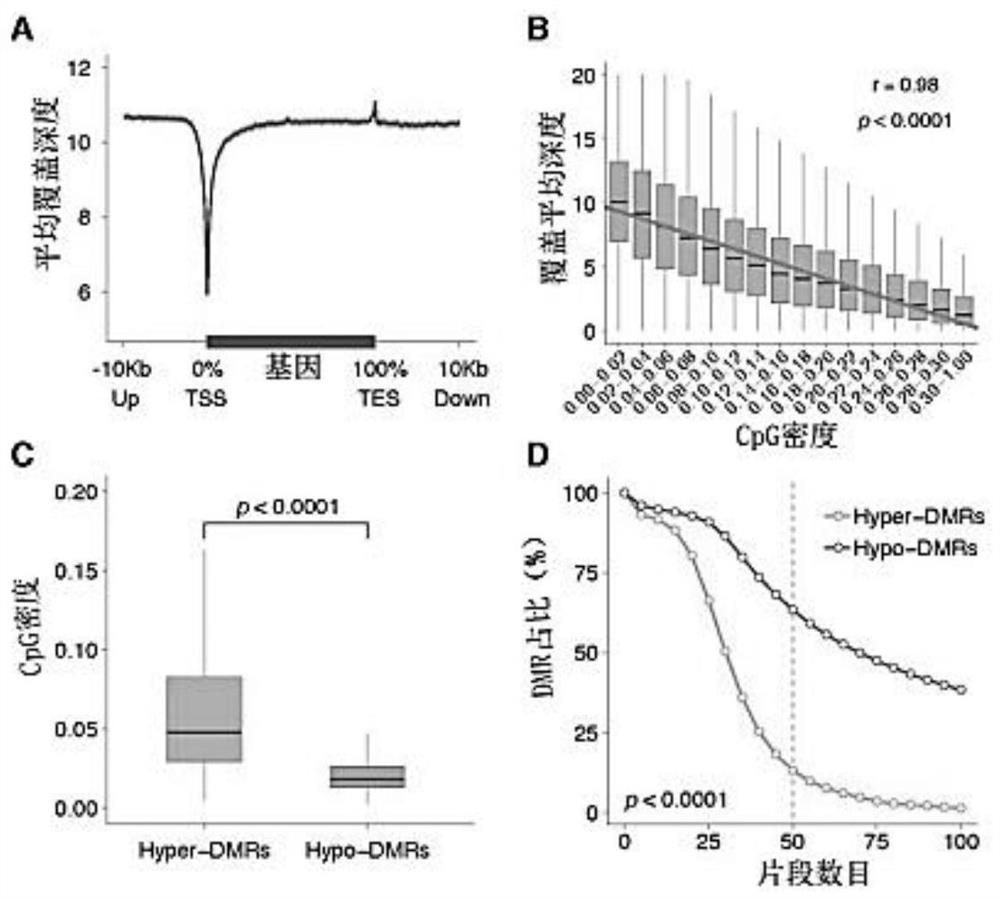
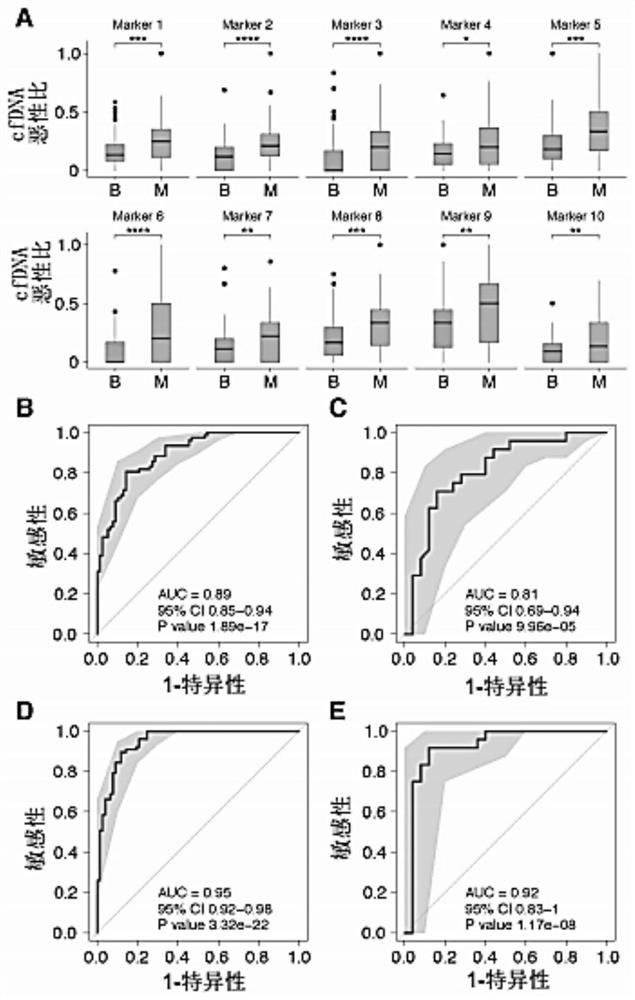
Application of cfDNA in non-invasive diagnosis of early breast cancer
ActiveCN111910004AAchieve early diagnosisNon-invasiveMicrobiological testing/measurementBiostatisticsScreening cancerEarly breast cancer
Application of cfDNA in non-invasive diagnosis of early breast cancer The present invention discloses an application of cfDNA in non-invasive diagnosis of early breast cancer, a cfDNA marker with relatively high sensitivity and specificity for diagnosing breast cancer and a product for diagnosing breast cancer prepared based on the marker, and at the same time a method for screening cancer markers.
Owner:WENZHOU INST UNIV OF CHINESE ACAD OF SCI +3
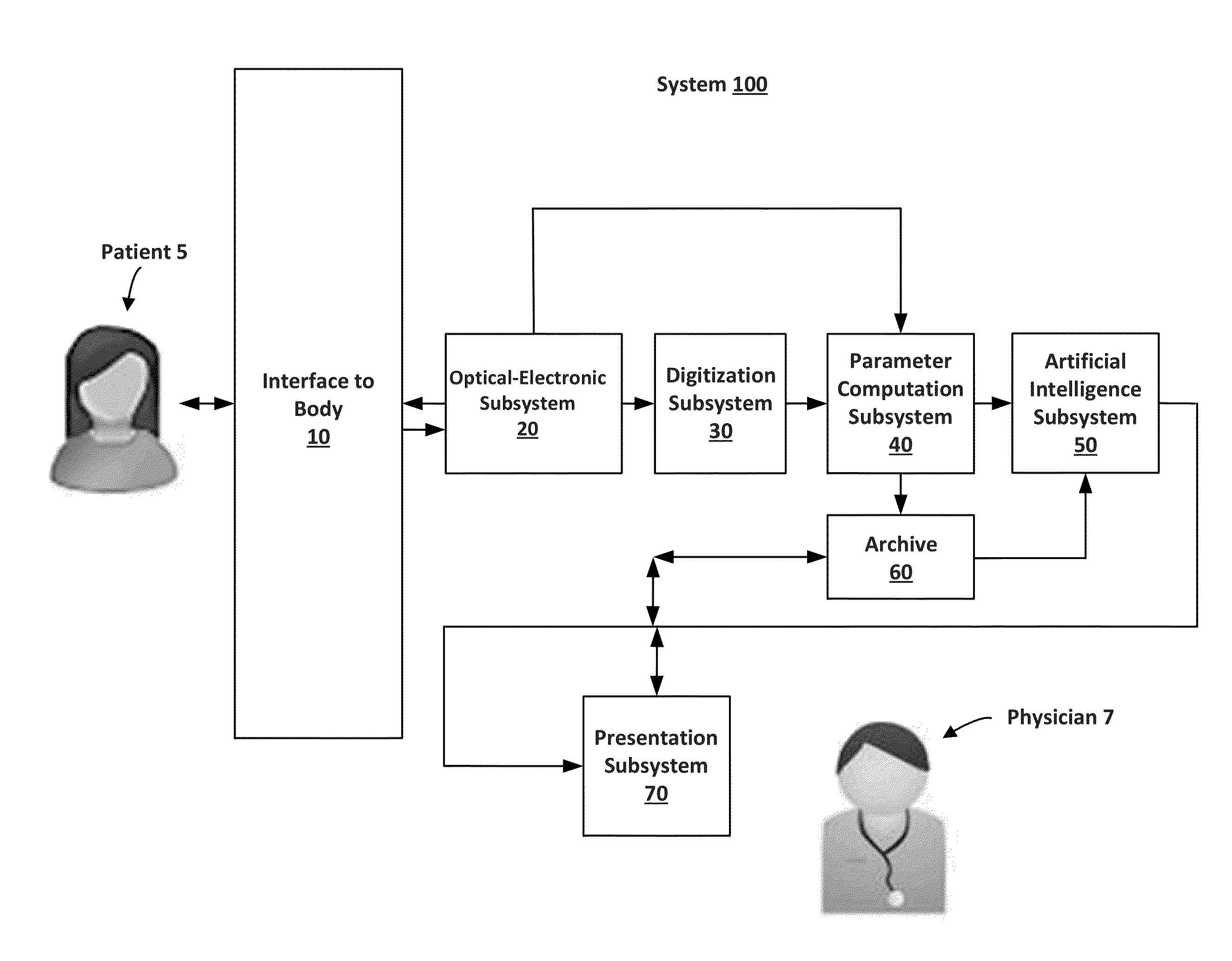
Portable cancer diagnostic device and system
InactiveUS20160235372A1Increase contrastOptimizes accuracy and reliabilityOrgan movement/changes detectionDiagnostics using spectroscopySpectroscopyEarly breast cancer
A portable device and system, based upon Diffuse Optical Spectroscopy (DOS), for the detection of surface detectable cancers such as breast cancer and the determination of their response to therapy. The system may include hardware and software components that form a number of subsystems: an Optical-Electronic Subsystem, a Digitization Subsystem, an Optical Parameter Computation Subsystem, an Artificial Intelligence Subsystem, and a Presentation Subsystem. The system can be integrated into a hybrid architecture that utilizes other imaging techniques, such as X-ray mammography, for cancer detection.
Owner:TELEBYTE
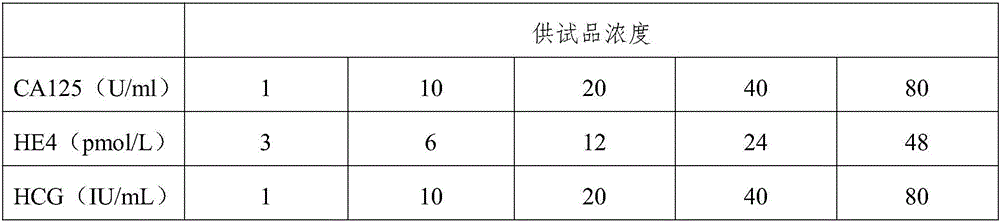
Triple test diagnostic kit for breast cancer
ActiveCN105866418AHigh sensitivityPlay the role of multi-stage amplificationMaterial analysisPositive controlEarly breast cancer
The invention belongs to the field of biological detection and specifically relates to a triple test diagnostic kit for breast cancer of tumor serum markers CA125, HE4 and P-her2. The triple test diagnostic kit for breast cancer provided by the invention mainly comprises an abzyme elisa plate, a biotin antibody enveloped plate, bovine serum albumin, serum diluent, horse radish peroxidase, tetramethyl benzidine, sulfuric acid, negative control liquid and positive control liquid. The triple test diagnostic kit for breast cancer provided by the invention can be used for detecting the expression levels of CA125, HE4 and P-her2 in the body of the patient after operative treatment or the relapsing and metastatic tumor patient; the triple test diagnostic kit for breast cancer has important significance in clinical diagnosis of breast cancer; the triple test diagnostic kit for breast cancer provided by the invention can reduce the interference of other matters in serum, can greatly increase the accuracy and stability of detection result and is beneficial to the early diagnosis and prognosis treatment of the patient suffering from breast cancer.
Owner:赛特斯(海南)生物医学有限公司
Grading of breast cancer
InactiveUS7930105B2Accurate assessmentDetermining prognosisOrganic active ingredientsSugar derivativesEarly breast cancerOncology
Methods and compositions for the identification of breast cancer grade signatures are provided. The signature profiles are identified based upon multiple sampling of reference breast tissue samples from independent cases of breast cancer and provide a reliable set of molecular criteria for identification of cells as being in one or more particular stages and / or grades of breast cancer.
Owner:THE GENERAL HOSPITAL CORP
Method for treating breast cancer and ovarian cancer
The present invention relates to a method for the prevention or treatment of certain breast cancers or ovarian cancer comprising administering to a patient in need thereof of a therapeutically effective amount of a 17,20-lyase inhibitor, wherein the breast cancer or ovarian cancer is estrogen receptor (ER) negative.
Owner:TAKEDA PHARMA CO LTD
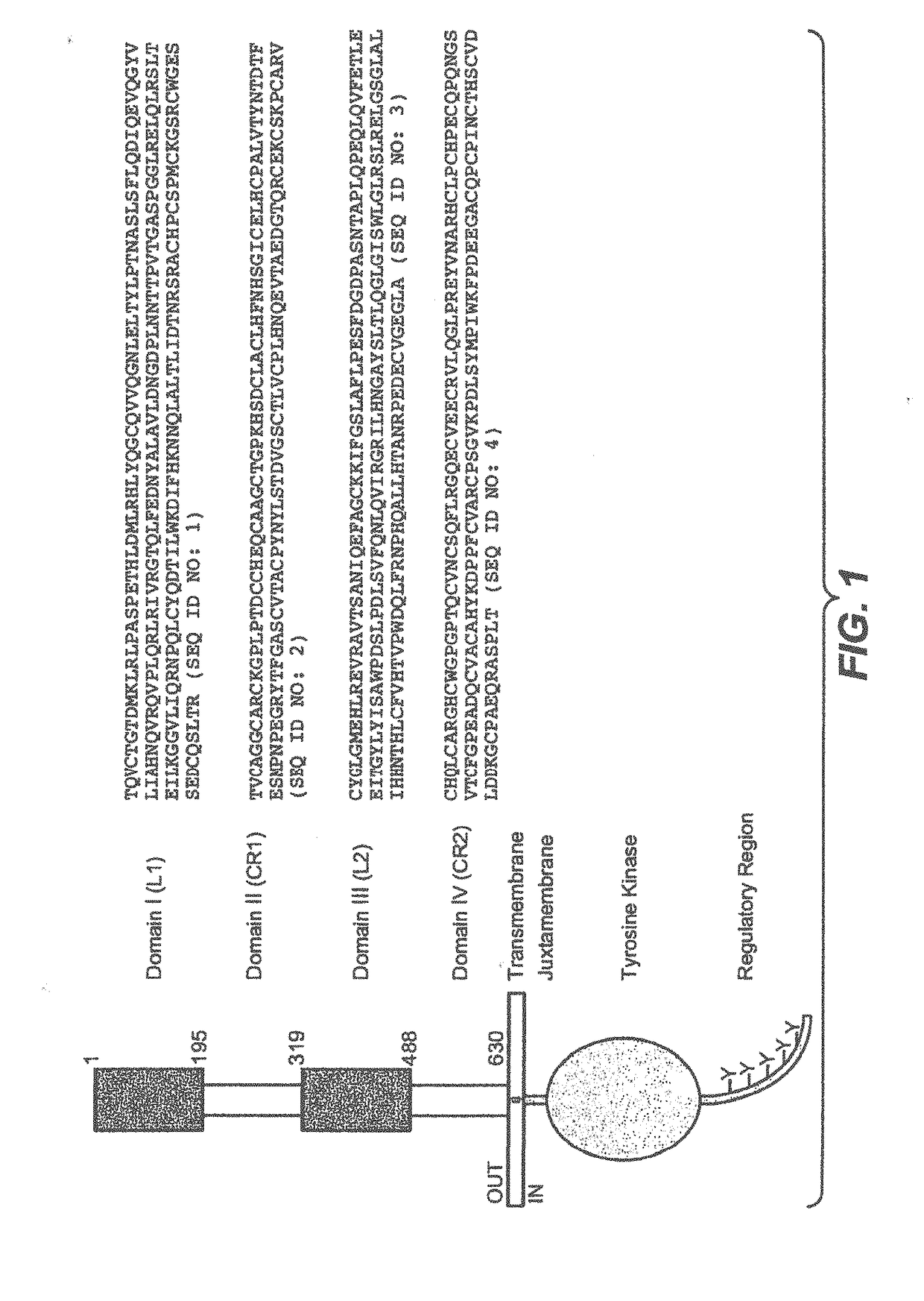
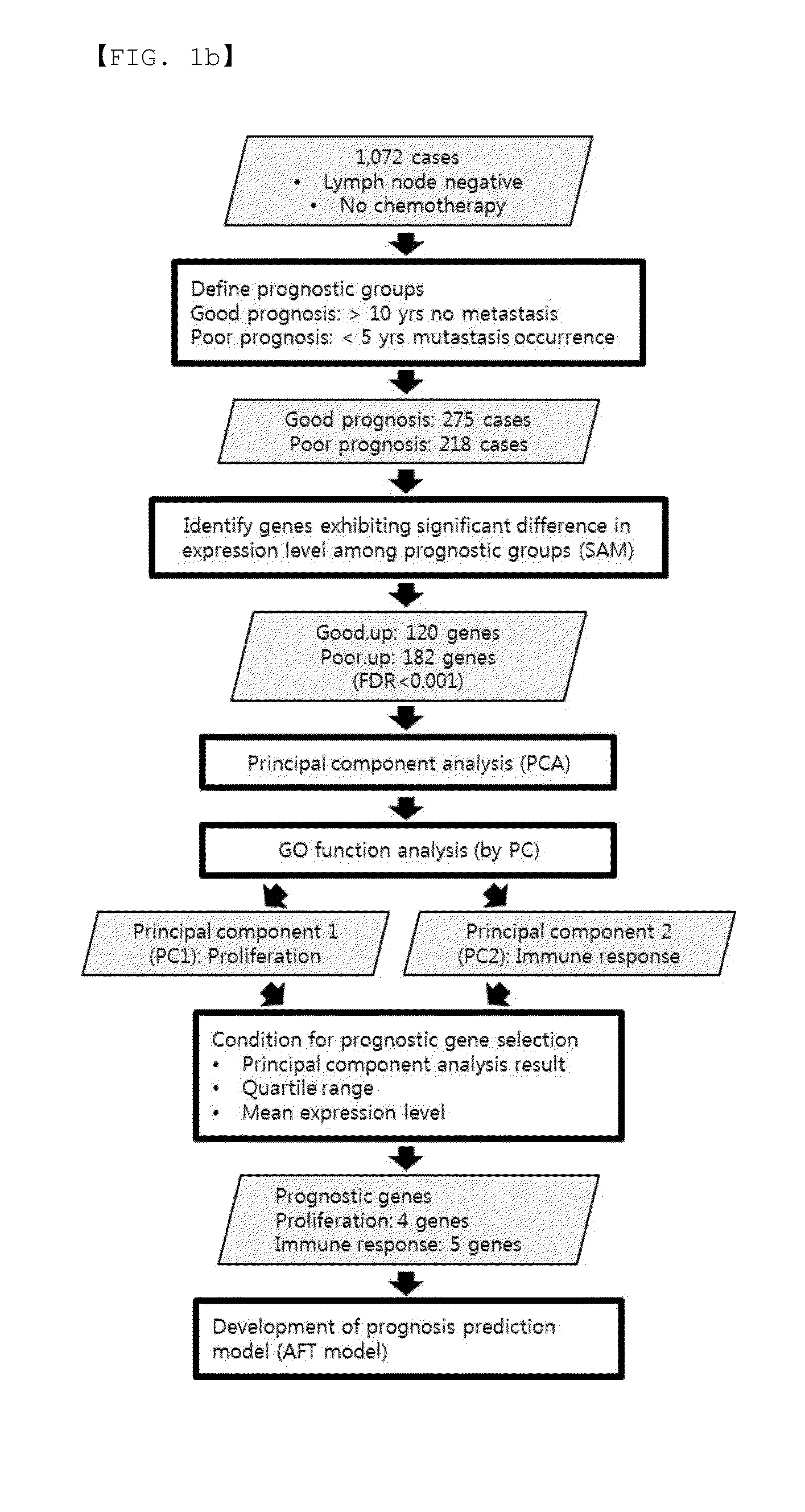
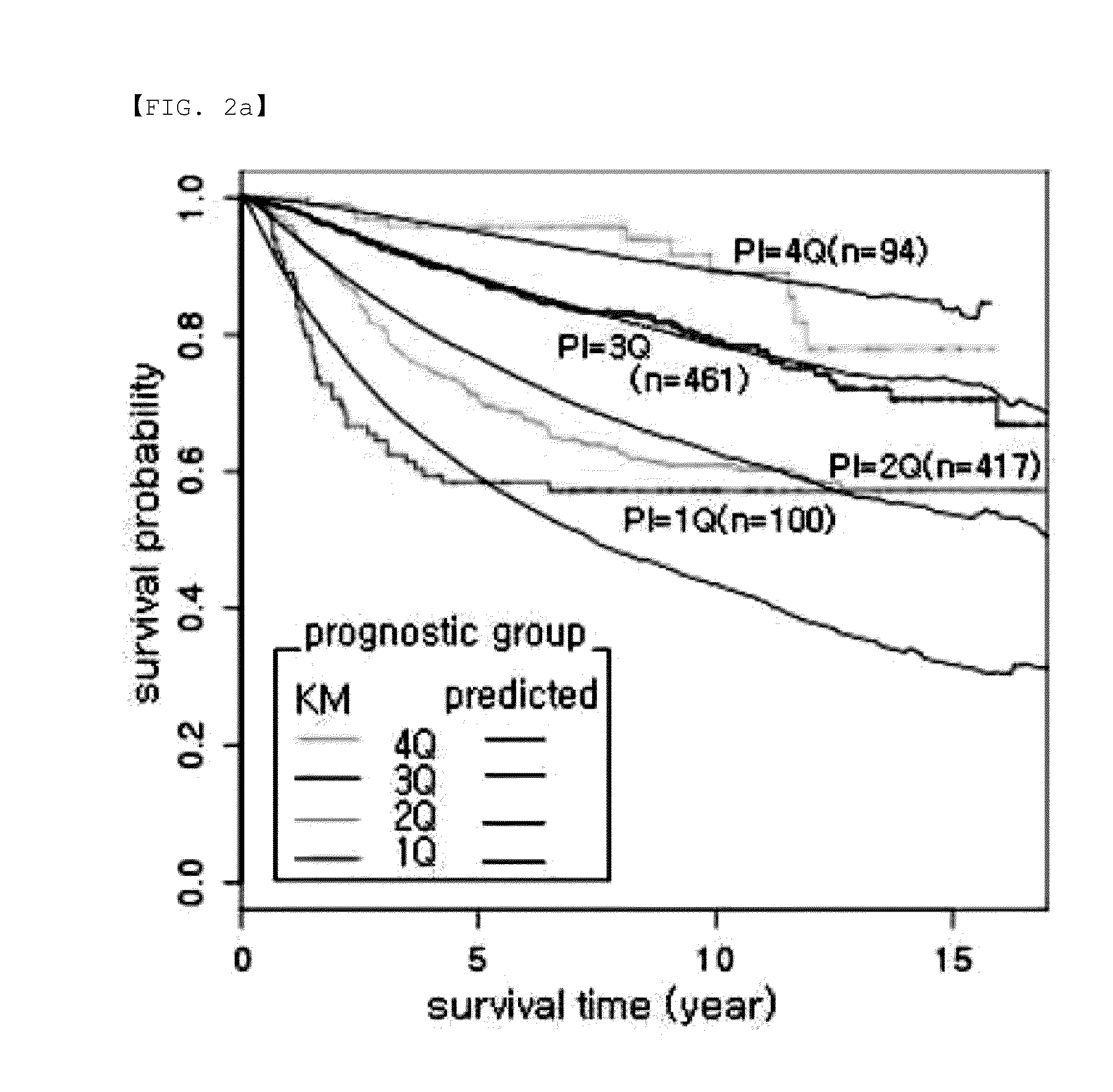
Genetic marker for early breast cancer prognosis prediction and diagnosis, and use thereof
InactiveUS20160102359A1Predict prognosisMicrobiological testing/measurementDisease diagnosisMajor histocompatibilityT-Cell Receptor Beta
A gene for predicting or diagnosing the prognosis of early-stage breast cancer and to a use thereof is disclosed, and more specifically a genetic marker for predicting or diagnosing the prognosis of breast cancer, including TRBC1 (T cell receptor beta constant 1), BTN3A2 (butyrophilin, subfamily 3 member A2) or HLA-DPA1 (major histocompatibility complex, class II, DP alpha 1) for providing information necessary for predicting or diagnosing the prognosis of a breast cancer patient. The genetic marker allows the prediction or diagnosis of the prognosis of a breast cancer patient, and can therefore advantageously be used for the purpose of providing a direction as to the future course of breast cancer treatment, including a decision on whether anticancer therapy is necessary.
Owner:GENCURIX
Method for the early detection of breast cancer, lung cancer, pancreatic cancer and colon polyps, growths and cancers as well as other gastrointestinal disease conditions and the preoperative and postoperative monitoring of transplanted organs from the donor and in the recipient and their associated conditions related and unrelated to the organ transplantation
ActiveUS7820382B2Early diagnosisMicrobiological testing/measurementDisease diagnosisEarly breast cancerLung cancer
A method for the early diagnosis of breast, lung, pancreatic and colon growths and cancers as well as conditions associated with donor and recipient organ transplants, both before and after transplantation to identify and allow treatment of possible transplanted organ rejection and other disease conditions related and unrelated to the transplantation, compares the gene expression patterns from a patient's peripheral blood monocytes-lymphocyte's gene system with either the similar gene expression patterns of a normal person, or with the similar gene expression patterns of a person known to have the condition being screened for. Differences between the patient's gene expression patterns for particular genes and the normal patterns indicates the presence of the condition with the number of differences indicating the probability of the condition. Similarities between the patient's gene expression patterns for those particular genes and the patterns of a person known to have the condition indicates the presence of the condition with the number of similarities indicating the probability of the condition. For example, particular genes for use in identifying pancreatic cancer are disclosed.
Owner:BAUER A ROBERT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com