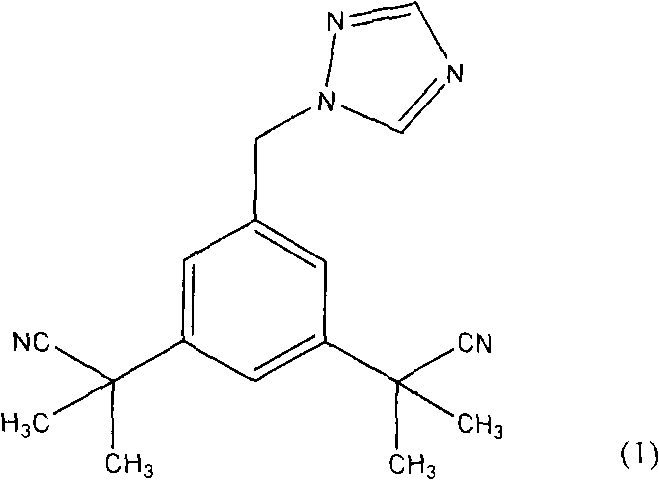
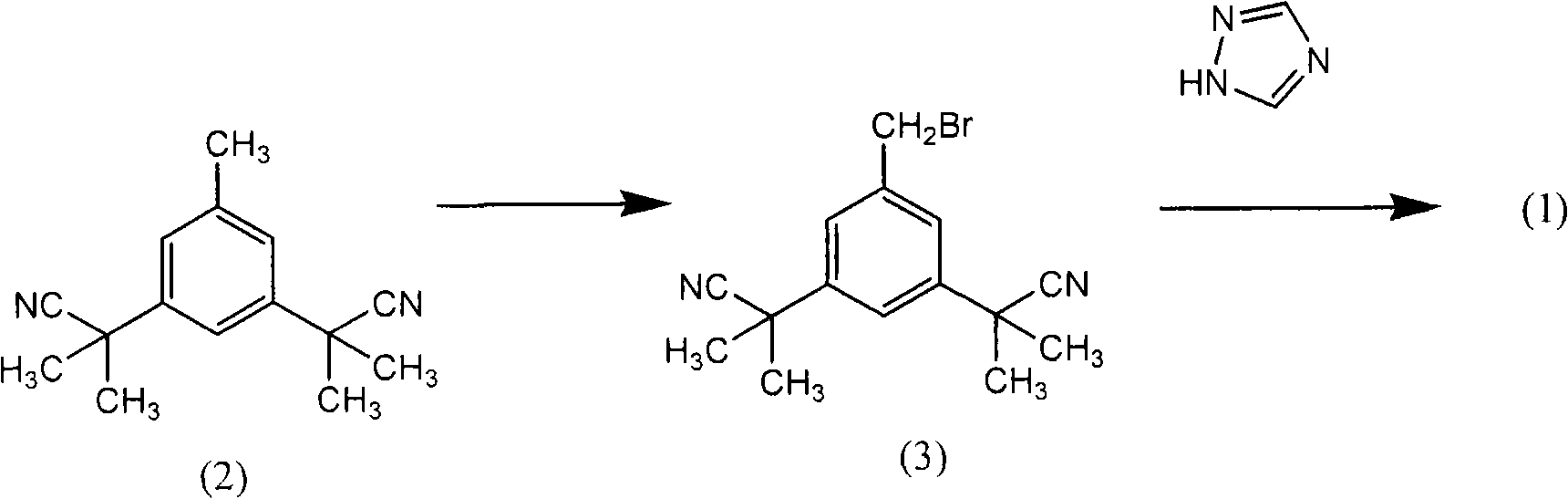
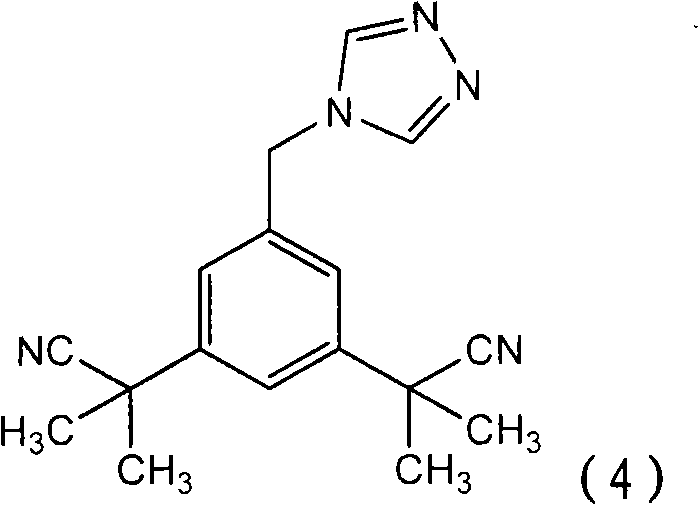
Process for purification of anastrozole
一种阿那曲唑、阿那曲唑碱的技术,应用在阿那曲唑的纯化领域,能够解决很难、除去杂质质量标准等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] [0034] In particular, the present invention can provide pharmaceutical grade anastrozole by using the method of the above invention in operative combination. Especially preferred are the following steps corresponding to the synthesis method of anastrozole.
[0058] [0035] The first step in the process is to provide a reaction mixture containing anastrozole. The reaction mixture comprising the anastrozole molecule is provided according to any known method, but preferably by condensation reaction of a bromomethyl compound of formula (3) or a chloromethyl compound of formula (6) with 1,2,4-triazole get. These starting materials are either commercially available or may be prepared by methods known in the art. Preferably the starting materials are used in a purified state. Although the purification of bromomethyl compounds of formula (3) is sufficiently disclosed in the prior art, no purification process for chloromethyl compounds of formula (6) has been found. However, ...
experiment Embodiment 1
[0079] Stability of Anastrozole Base in Dilute Hydrochloric Acid Aqueous Solution
[0080] [0054] 0.5 g of anastrozole base was suspended in 10 ml of distilled water at room temperature and stirred, and 0.155 ml (1.1 equivalent) of concentrated hydrochloric acid was added under stirring. The mixture was stirred at room temperature for 1 hour, and the solid was filtered and washed with 5 ml of water. Dry in air at room temperature for 2 days.
[0081] Melting point (capillary) 82.6°C.
[0082] Structure: Anastrozole Base
experiment Embodiment 2
[0084] Conversion of anastrozole base to anastrozole hydrochloride in excess of hydrochloric acid
[0085] [0055] 0.5 g of anastrozole base was suspended in 5 ml of 10% aqueous hydrochloric acid at room temperature with stirring. Some of the solids dissolved. For complete dissolution, a further 3 ml of the same acid were added and the mixture was heated to 42°C. The solution was filtered and the filtrate was washed with 2 ml of the same acid. The solution was treated with 10 ml of distilled water with stirring at 20-25°C and stirred for 30 minutes. The solution became cloudy and the crystalline product separated. The mixture was cooled to 5°C and stirred for 1 hour. The solid was filtered, washed with 5ml of water and air dried.
[0086] Yield: 0.633 g of Anastrozole hydrochloride (dihydrate)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com