Applications of combined CDK4/6 inhibitor and aromatase inhibitor in preparing medicines for treating breast cancer
An aromatase inhibitor and aromatase technology, which can be used in drug combination, organic chemistry, antitumor drugs, etc., and can solve the problems of no specific disclosed effect and no expected synergistic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
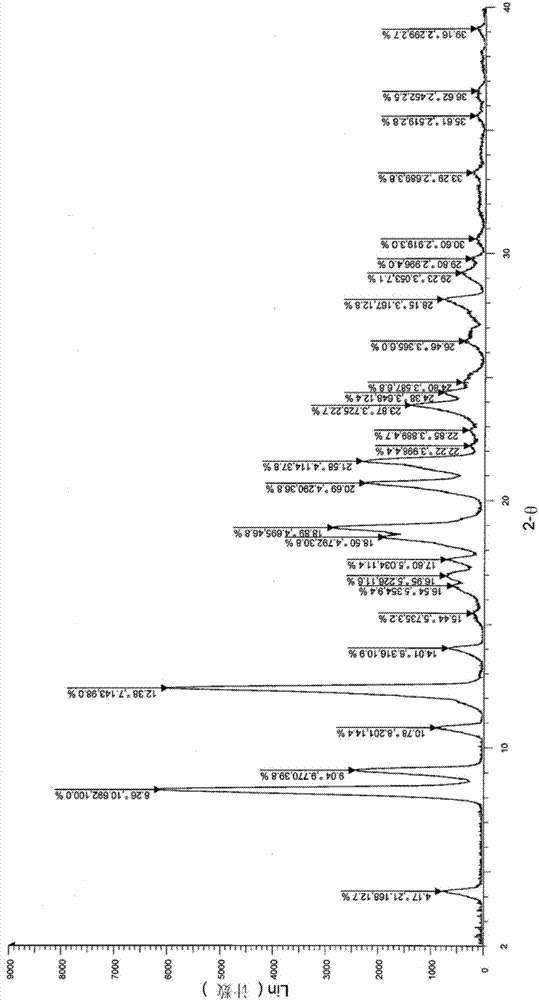
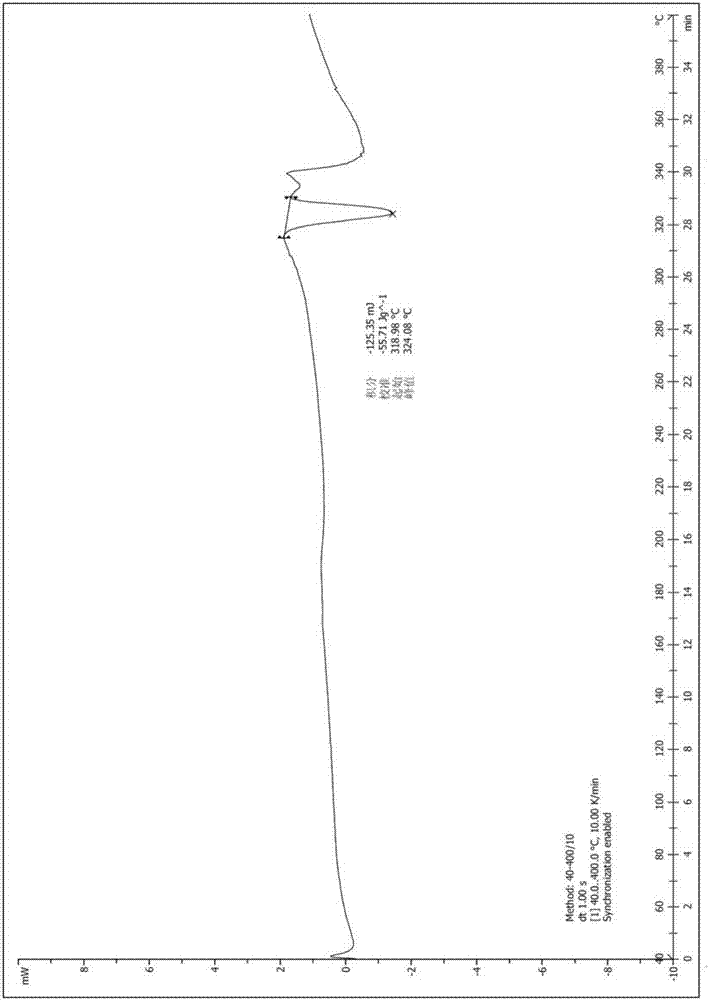
[0036] Example 1: 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperidin-4-yl)pyridin-2-yl)amino)pyrido[2,3-d ]Pyrimidin-7(8H)-One Preparation of Isethionate
[0037] Step 1: 6-((6-(1-butoxyethenyl)-8-cyclopentyl-5-methyl-7-carbonyl-7,8-dihydropyrido[2,3-d]pyrimidine Preparation of -2-yl)amino)-5',6'-dihydro-[3,4'-bipyridine]-1'(2'H)-tert-butyl formate
[0038]
[0039] Under argon protection, (10g, 29.06mmol) 2-amino-6-(1-butoxyethenyl)-8-cyclopentyl-5-methylpyrido[2,3-d]pyrimidine-7( 8H)-ketone (prepared according to the method disclosed in WO2014183520), cesium carbonate (14.22g, 43.75mmol), Pd 2 (dba) 3 (2.12g, 2.31mmol), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (2.69g, 4.69mmol) and 125.00g of dioxane were put into a three-necked reaction flask and stirred evenly After heating to reflux, the raw material 4-(6-chloropyridin-3-yl)-5,6-dihydropyridine-1(2H)-tert-butyl carboxylate (10.34g, 35.00mmol, purchased from Yancheng City Ruikang Pharmaceutical Chemical Co., Ltd...
Embodiment 2
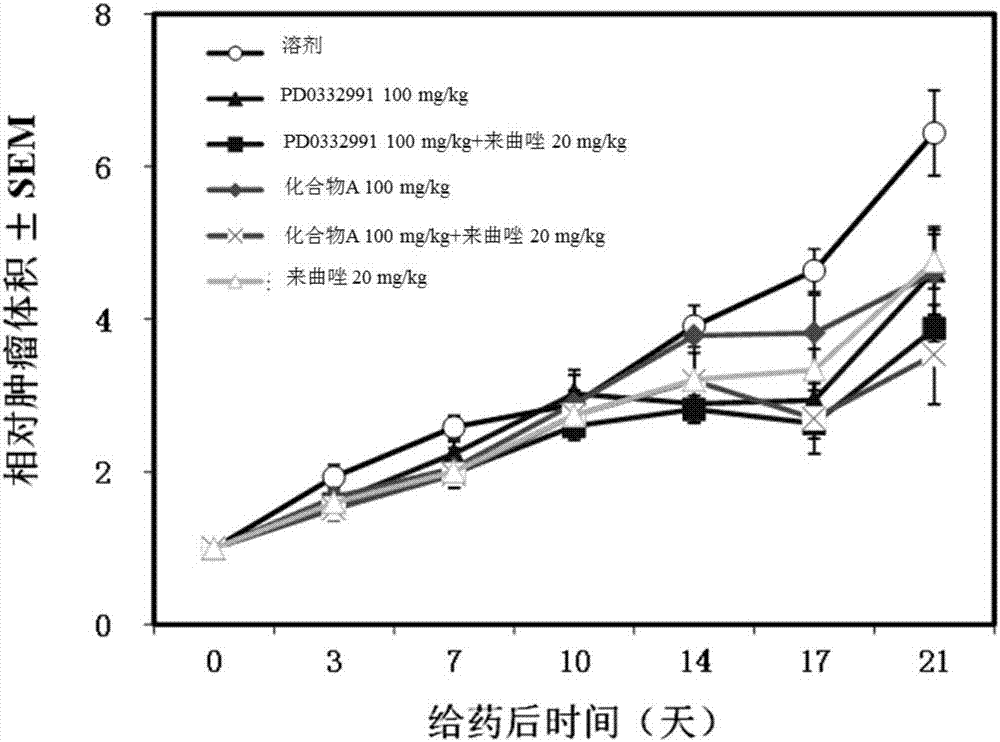
[0046] Example 2: Comparative study on the curative effect of compound A and PD-0332991 alone or in combination with letrozole on human breast cancer MCF-7 / ARO subcutaneously transplanted tumors in nude mice.
[0047] 1 test drug
[0048] Drug name: the isethionate of PD-0332991 is a yellow crystalline powder, prepared according to the method disclosed in CN1835951B; the isethionate of compound A is a white powder, prepared from Example 1; letrozole is White powder, provided by Jiangsu Hengrui Pharmaceutical Co., Ltd.
[0049] Preparation method: the isethionate of compound A was prepared with 50mM citric acid + 0.5% CMC + 0.5% Tween80; the isethionate of PD-0332991 was prepared with distilled water containing 0.1% Tween 80; letrozole was prepared with Prepare and dilute with 20% PEG 400 in distilled water.
[0050] 2 experimental animals
[0051] BALB / cA-nude nude mice, 6-7 weeks old, ♀, were purchased from Shanghai Lingchang Biotechnology Co., Ltd. Production license num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com