Preparation method for recombinant human interferon alpha-2b free of methionine
A technology of recombinant human interferon and methionine, applied in the directions of interferon, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problem of affecting interferon activity, low yield, increasing the incidence of adverse reactions, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] The experimental methods in the following examples are conventional methods unless otherwise specified.
[0034] The percentages in the following examples are all mass percentages unless otherwise specified.
[0035] Below in conjunction with embodiment the present invention is described in detail.
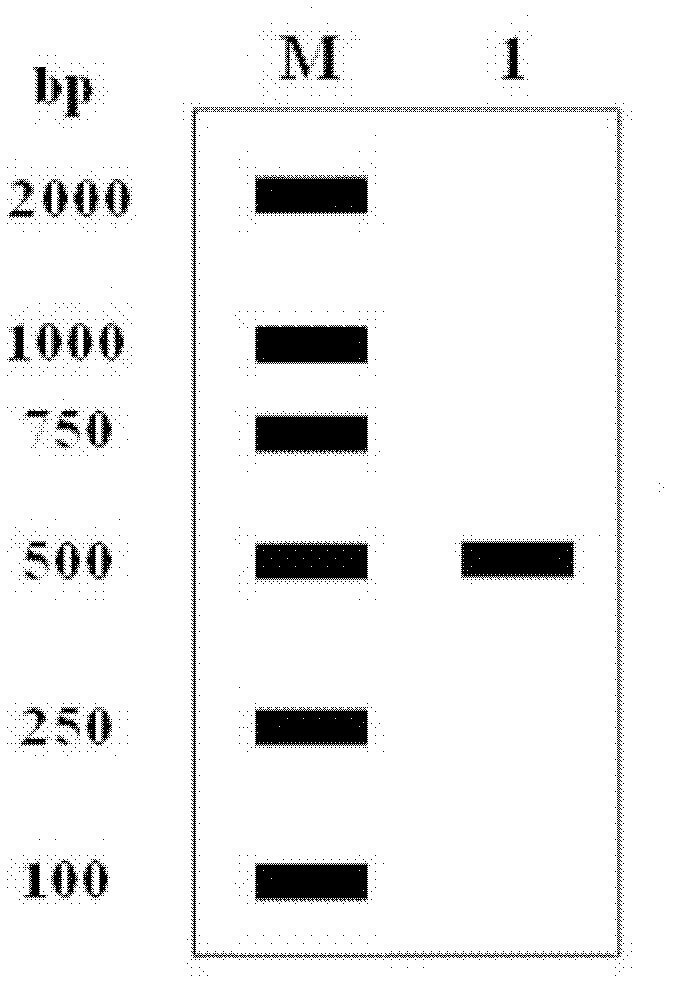
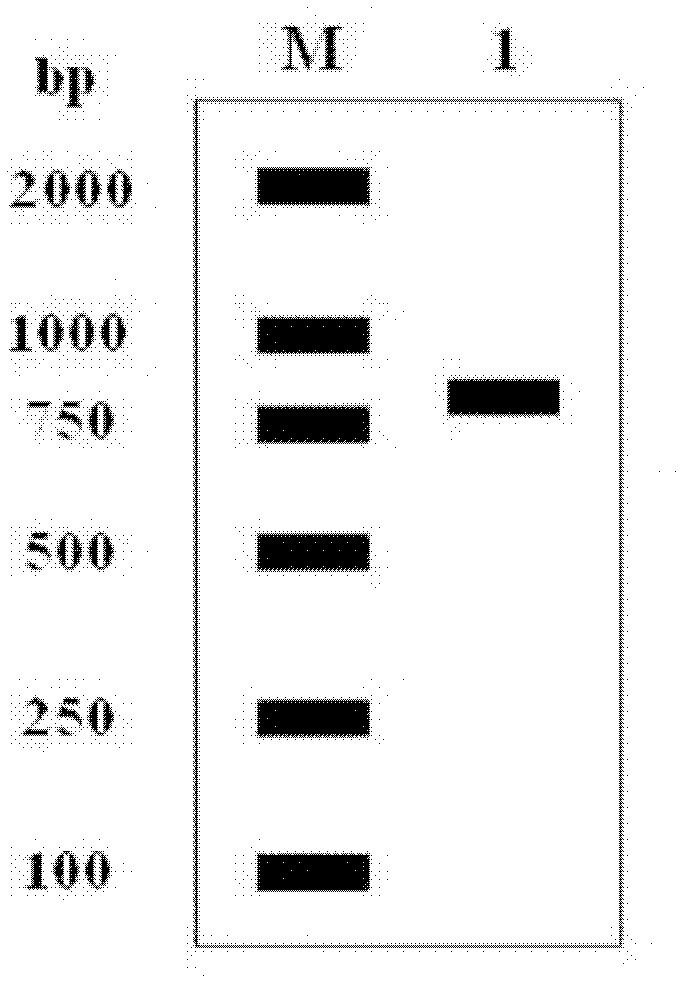
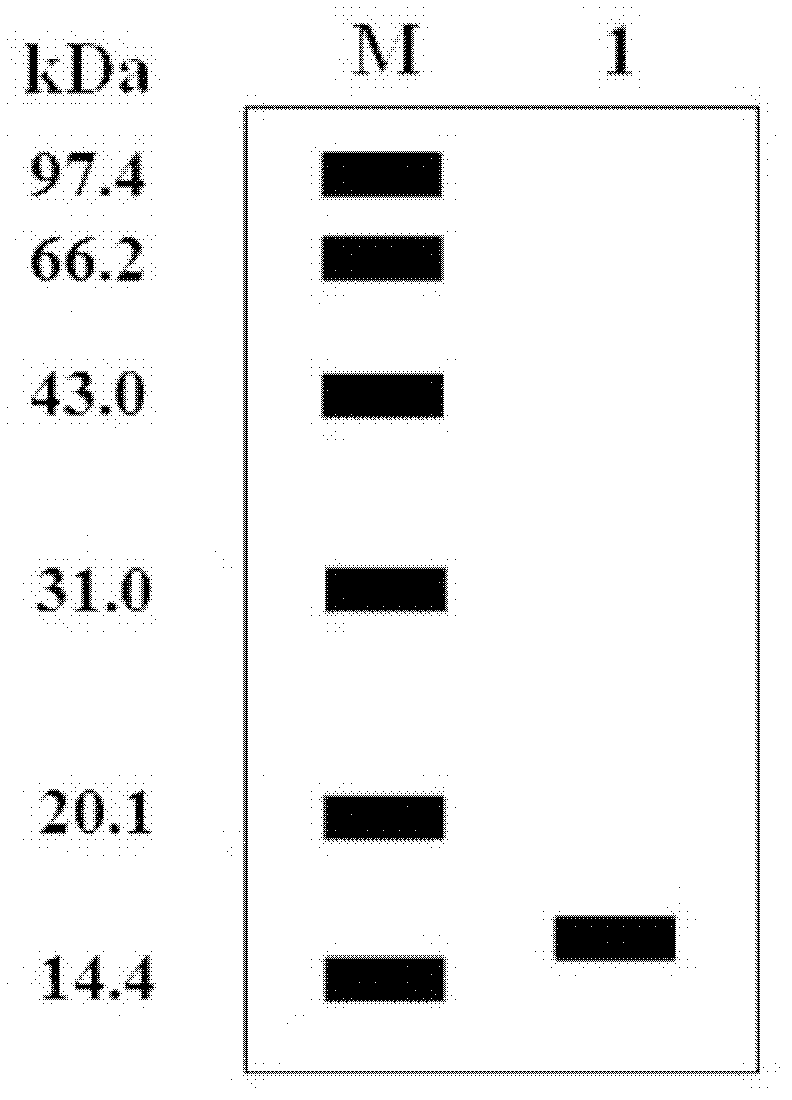
[0036] 1. Construction of recombinant human interferon-α2b expression vector
[0037] Design upstream and downstream primers P1 and P2:
[0038] P1: GG CATATG TGTGATCTGCCTCAAACCCAC
[0039] P2: GG GGATCC TTATTCCTTACTTCTTAAACTTTC
[0040] The underlined parts of P1 and P2 are Nde I and BamH I restriction sites, respectively.
[0041] Using the hIFN-α2b gene as a template, it was amplified by Polymerase Chain Reaction (PCR), and the PCR reaction program was: 94°C, 2min pre-denaturation; 94°C for 45s, 65°C for 45s, 72°C for 1min for 30 reactions Cycle; extend at 72°C for 10min; store at 4°C. The 50μl reaction system is: 10×Pfu DNA polymerase buffer (containing Mg 2+ ) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com