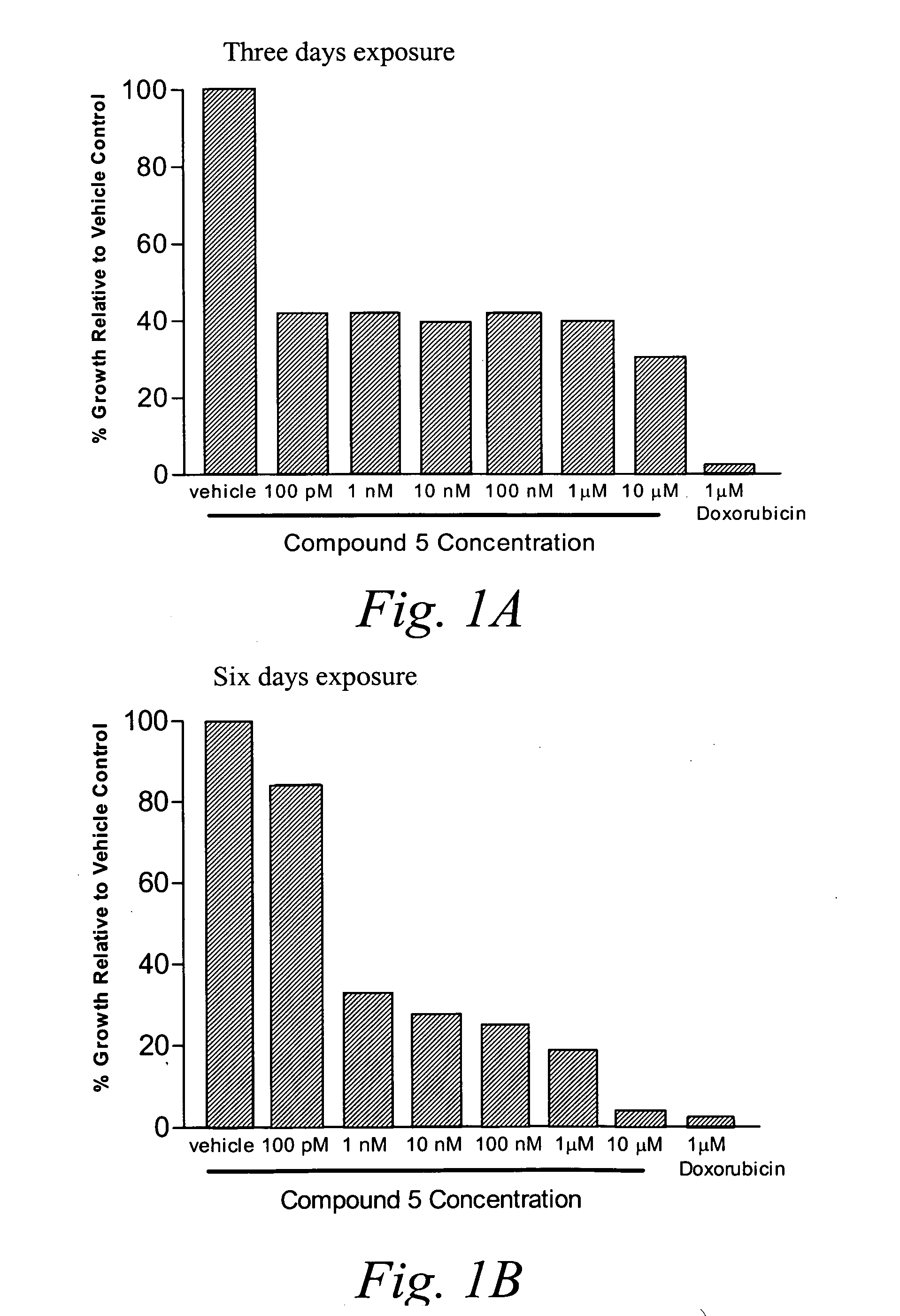
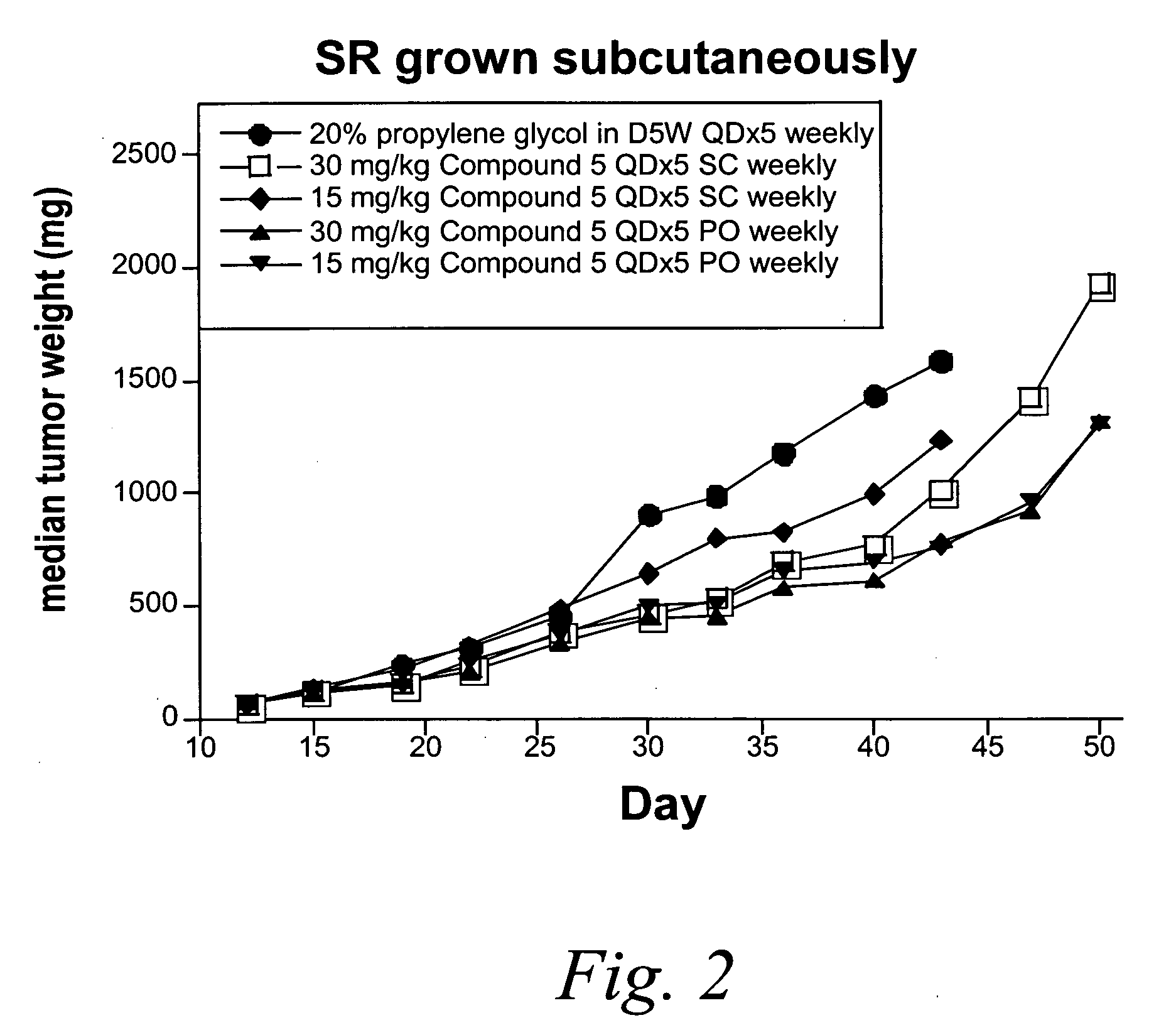
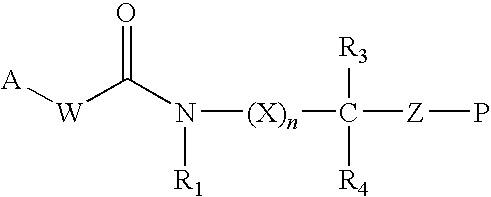
Methionine aminopeptidase-2 inhibitors and methods of use thereof
a technology of aminopeptidase and inhibitor, which is applied in the direction of peptides, drug compositions, immunological disorders, etc., can solve the problems of autoimmune disorders, all mechanisms have a risk of breakdown, and abnormal cells, so as to prevent metabolic degradation, reduce side effects, and improve the effect of pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-{(3R,4S,5S,6R)-5-Methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl-but-2-enyl)-oxiranyl]-1-oxa-spiro[2.5]oct-6-yloxycarbonylamino}-3-methyl-butyric Acid Methyl Ester
[0102]
[0103]General procedure A was followed using 1 (31 mg, 0.07 mmol), L-valine methyl ester hydrochloride (58 mg, 0.35 mmol), and DIEA (60 μL, 0.35 mmol) in EtOH (2 mL). Purification via flash chromatography (1% MeOH / CH2Cl2) afforded the product as a clear oil (10 mg, 0.02 mmol, 33% yield); Rf=0.60 (20% EtOAc / CH2Cl2); LRMS (m / z) [M+1]+ 440.3 (calculated for C23H38NO7, 440.3).
example 2
2-{(3R,4S,5S,6R)-5-Methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl-but-2-enyl)-oxiranyl]-1-oxa-spiro[2.5]oct-6-yloxycarbonylamino}-3-methyl-butyric Acid Methyl Ester
[0104]
[0105]General procedure A was followed using 1 (41 mg, 0.09 mmol) and D-valine methyl ester hydrochloride (77 mg, 0.45 mmol), and DIEA (80 μL, 0.45 mmol) in EtOH (2 mL). Purification via flash chromatography (1% MeOH / CH2Cl2) afforded the product as a clear oil (18 mg, 0.04 mmol, 45% yield); Rf=0.39 (20% EtOAc / CH2Cl2; LRMS (m / z) [M+1]+440.3 (calculated for C23H38NO7, 440.3).
example 3
2-{(3R,4S,5S,6R)-5-Methoxy-4-[(2R,3R)-2-methyl-3-(3-methyl-but-2-enyl)-oxiranyl]-1-oxa-spiro[2.5]oct-6-yloxycarbonylamino}-4-methyl-pentanoic Acid Methyl Ester
[0106]
[0107]General procedure A was followed using 1 (23 mg, 0.05 mmol), D-leucine methyl ester hydrochloride (47 mg, 0.25 mmol), and DIEA (45 μL, 0.25 mmol) in EtOH (2 mL). Purification via flash chromatography (1% MeOH / CH2Cl2) afforded the product as a clear oil (19 mg, 0.04 mmol, 83% yield); Rf=0.22 (15% EtOAc / CH2Cl2); LRMS (m / z) [M+1]+ 454.3 (calculated for C24H40NO7, 454.3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com