Use of A3 Adenosine Receptor Agonist in Osteoarthritis Treatment
a technology of adenosine receptor and osteoarthritis, applied in the field of treatment, can solve the problems of a large number of joints affected, a higher frequency of hip joint invasion, and a weak muscle strength, so as to prevent the development of osteoarthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
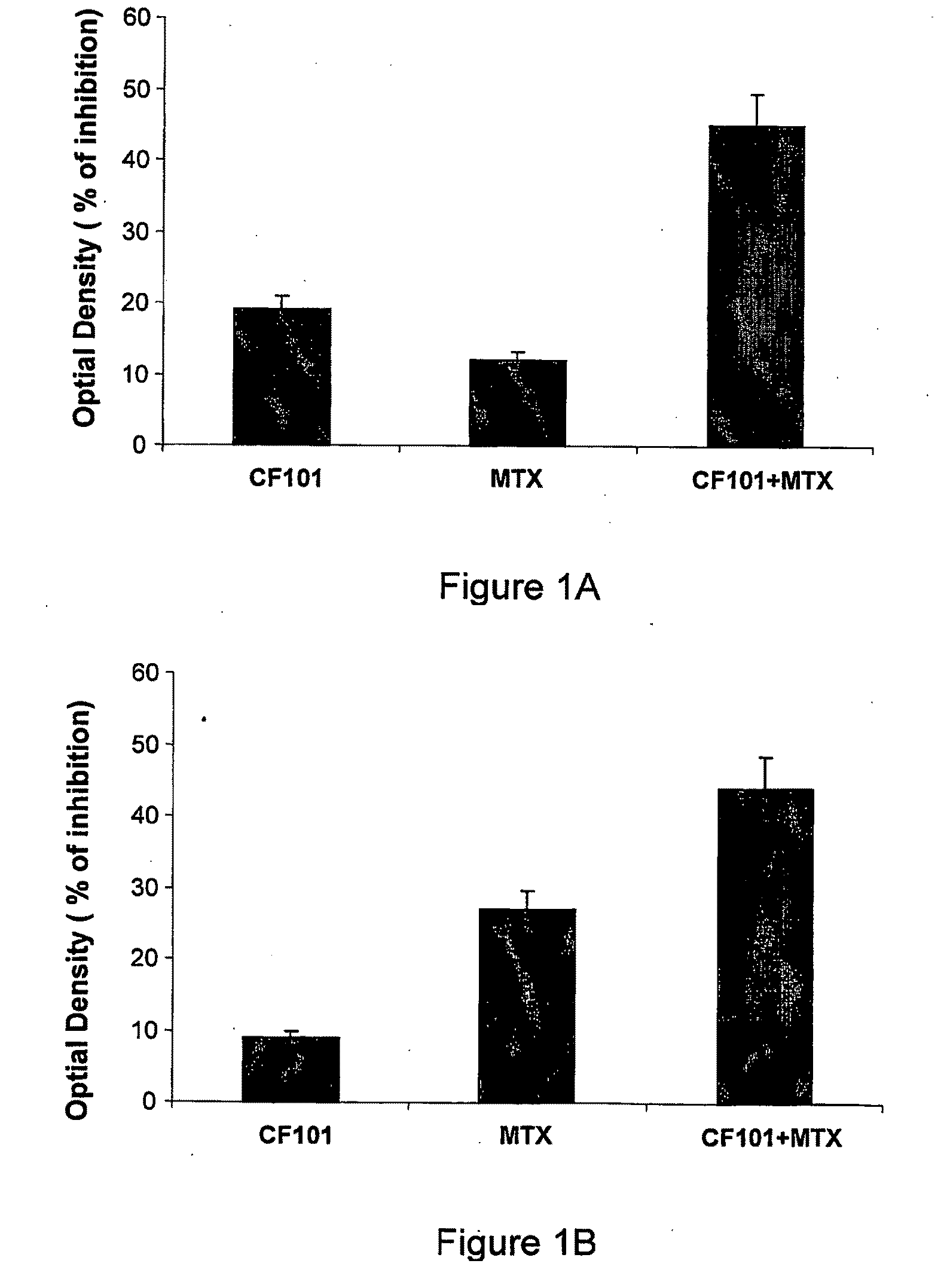
[0146]The effect of IB-MECA (herein at times CF101) alone or in combination with MTX, on the proliferation of the human or rat fibroblast like synoviocytes (FLS) was tested. An effect on proliferation of FLS is suggestive of potential therapeutic effect in osteoarthritis.
Human FLS Cultures
[0147]Human synovial fluid samples were collected from osteoarthritis (OA) patients undergoing paracenthesis. The fluid was centrifuged and the supernatant removed. The cells were resuspended in DMEM containing type I collagenase (4 mg / ml), for 2 hours, and shacked vigorously at 37°. The released cells in the supernatant were harvested by centrifugation and were cultured in DMEM containing 10% FBS, 2 mM glutamine, 100 U / ml penicillin, 100 μg / ml streptomycin, 1% non essential amino acids, 1% sodium pyruvate and 20 mM HEPES buffer in a 37° C., 5% CO2 incubator. After overnight culture, non-adherent cells were removed. The adherent cells (FLS) were subcultured at a 1:2 ratio, and the cells from passag...
example 2
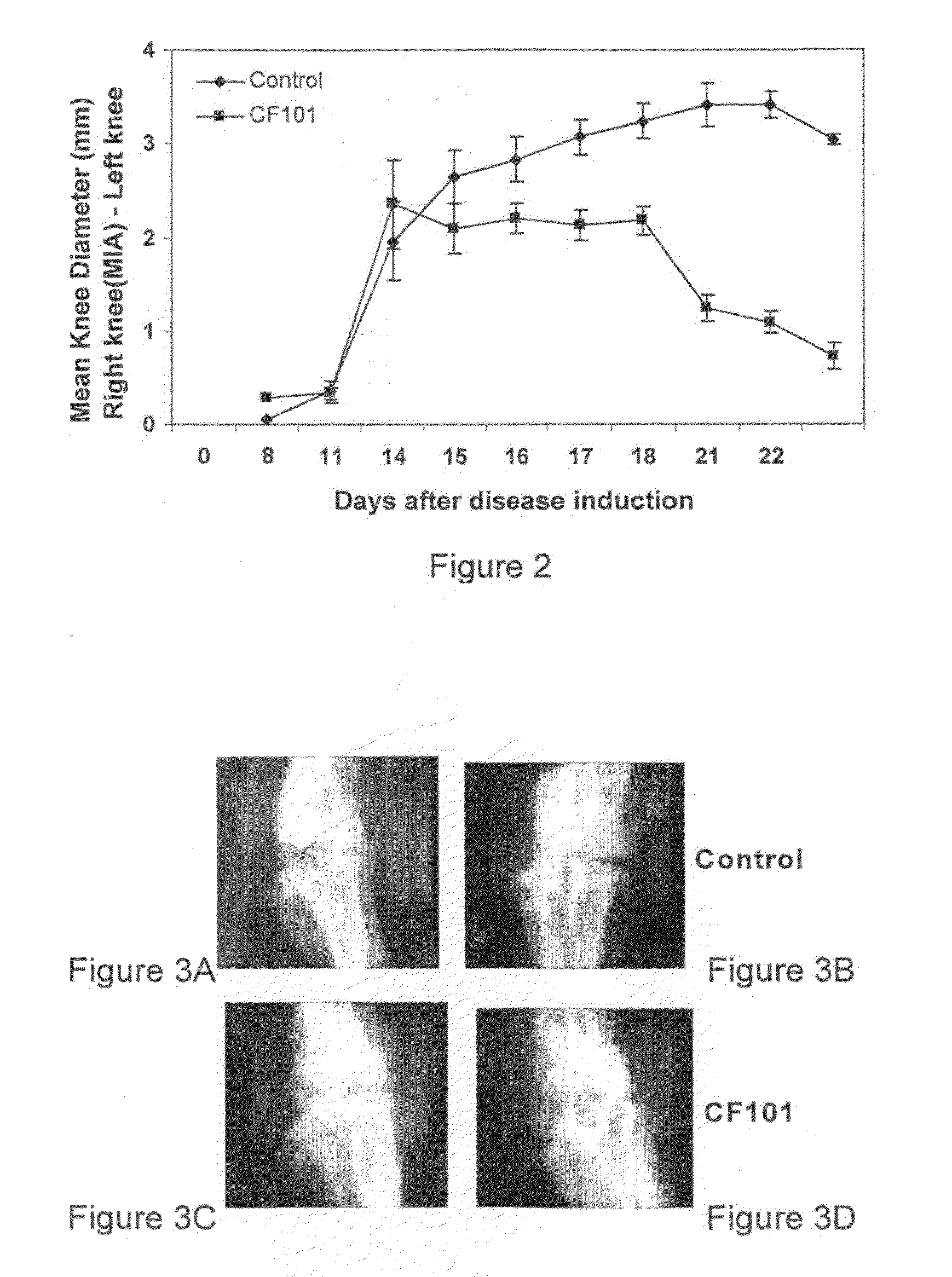
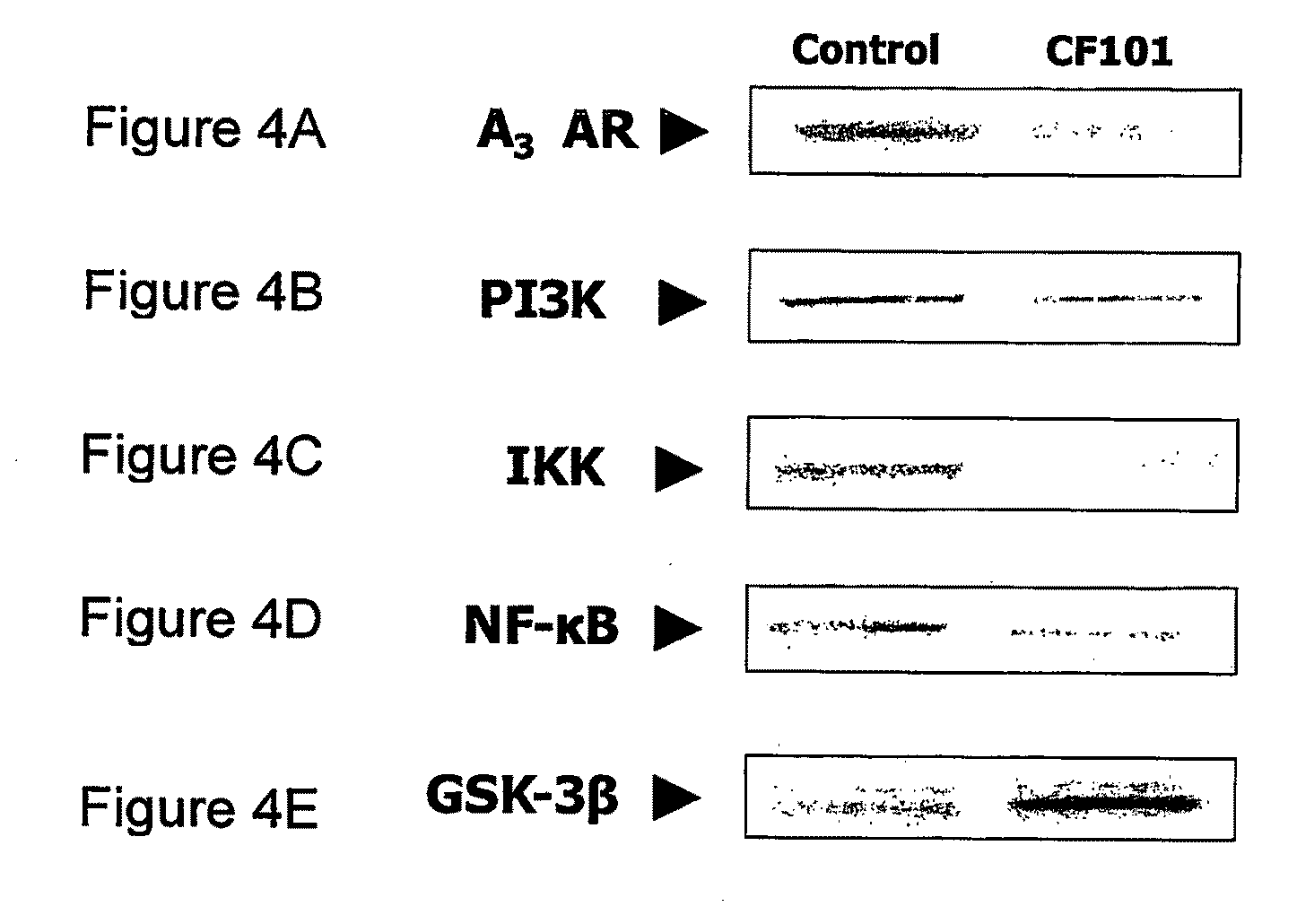
[0152]In the following study the effect of 13-ME CA (herein, at times, CF101) on the development of experimental osteoarthritis (OA) was determined. In this study the monoidoacetate (MIA) experimental model was utilized. The MIA is a rat experimental model that rapidly reproduces the clinical and pathological characteristic of OA. MIA is an inhibitor of glycolysis which has been shown to induce chondrocyte death in vitro. Intraarticular injection of MIA induces chondrocyte death in the articular cartilage of rodent.
[0153]Specifically, male Wistar rats (˜200 gr) (Harlan laboratories) were anesthetized with Isoflurane and the right leg was flexed at a 90° angle of the knee. The MIA was dissolved in physiological saline and 2 mg, at a volume of 50 μL were injected intraarticular of the right foot of each animal, using a 27-gauge, 0.5-inch needle.
[0154]Treatment with CF101, 100 μg / kg, Per Oz (PO), twice daily was initiated at day 7 post-injection.
[0155]Knee diameter was measured using c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com