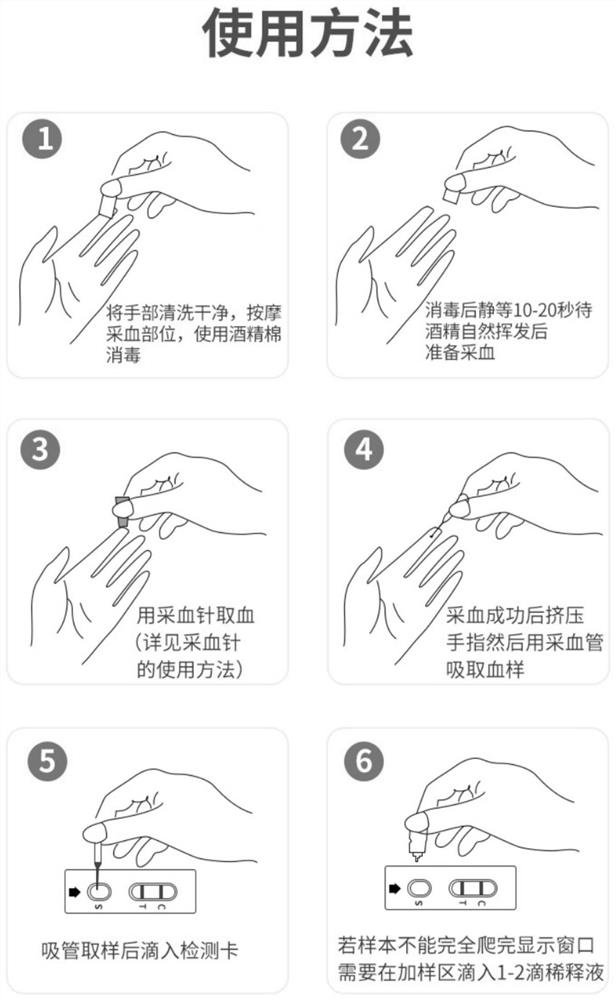
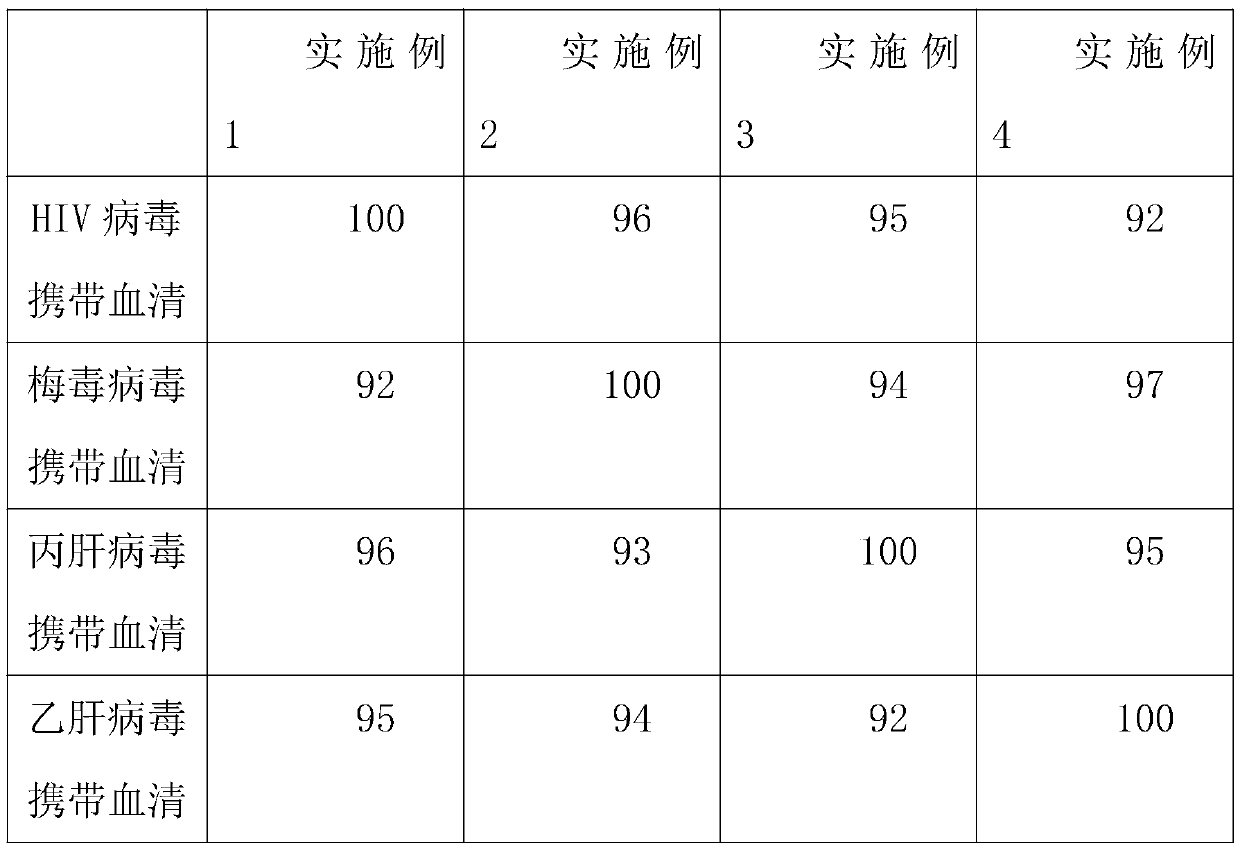
Patents
Literature
34 results about "HIV positives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-lethal conditioning methods for the treatment of acquired immunodeficiency syndrome
InactiveUS6039684AGood conditionPromote resultsPeptide/protein ingredientsEnergy modified materialsAcquired immunodeficiencyImmunodeficiency virus
The present invention relates to the use of a non-lethal preparative regimen for the treatment of a patient with human immunodeficiency virus (HIV) disease. In a clinical trial, an HIV-positive patient was conditioned by non-lethal doses of irradiation and a chemotherapeutic drug prior to receiving donor bone marrow cells from a baboon. While long-term engraftment of donor cells was not observed, the non-lethal preparative conditioning regimen was able to reduce the viral burden and improved the clinical outcome. Such method is useful for treatment of patients with advanced acquired immunodeficiency syndrome (AIDS).
Owner:ALLEGHENY UNIV OF THE HEALTH SCI +1
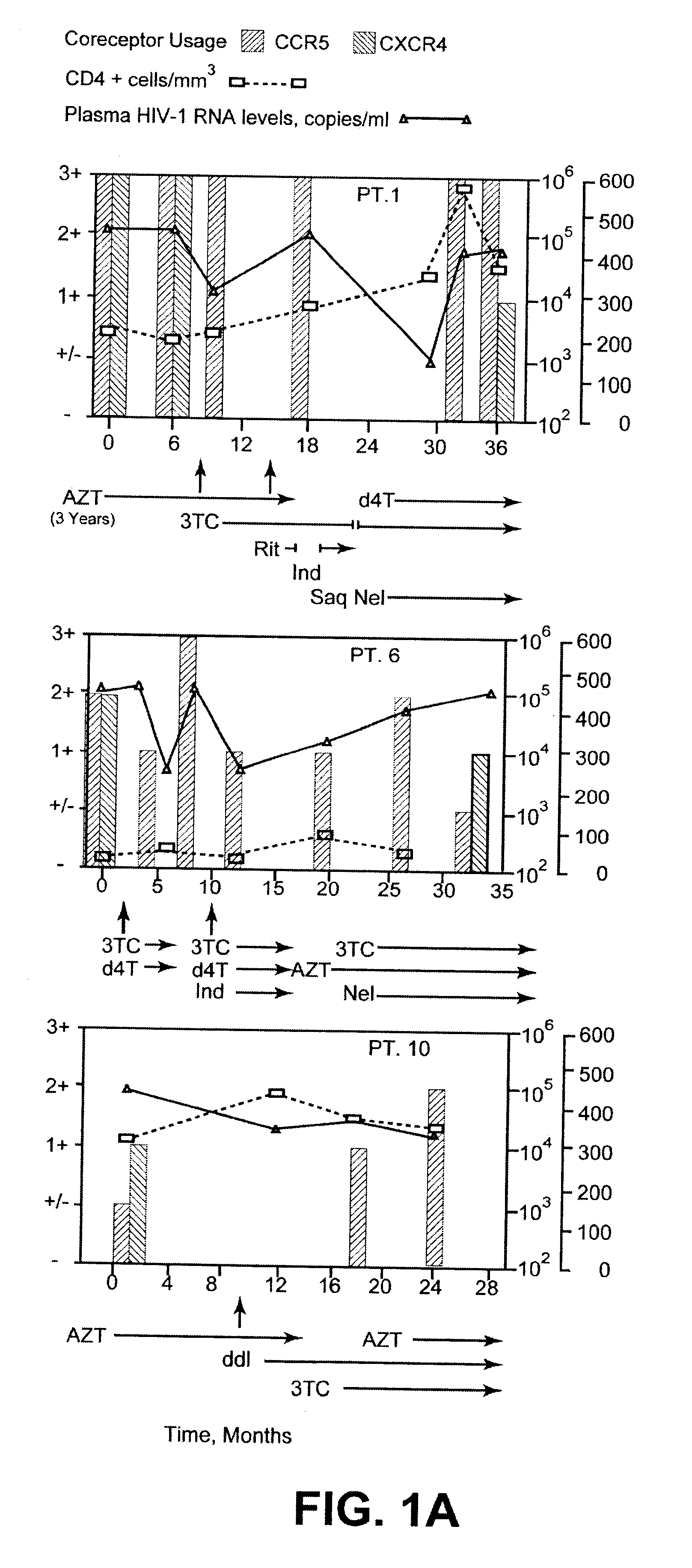
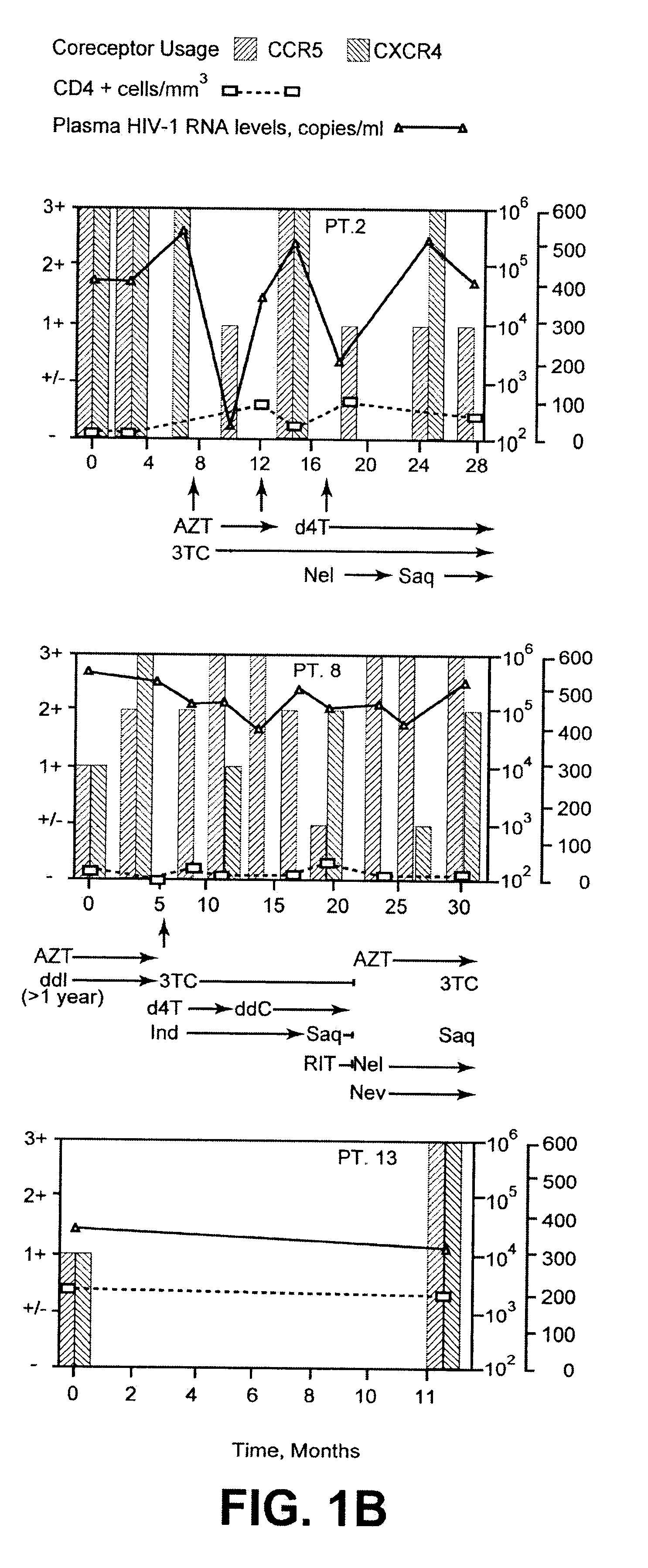
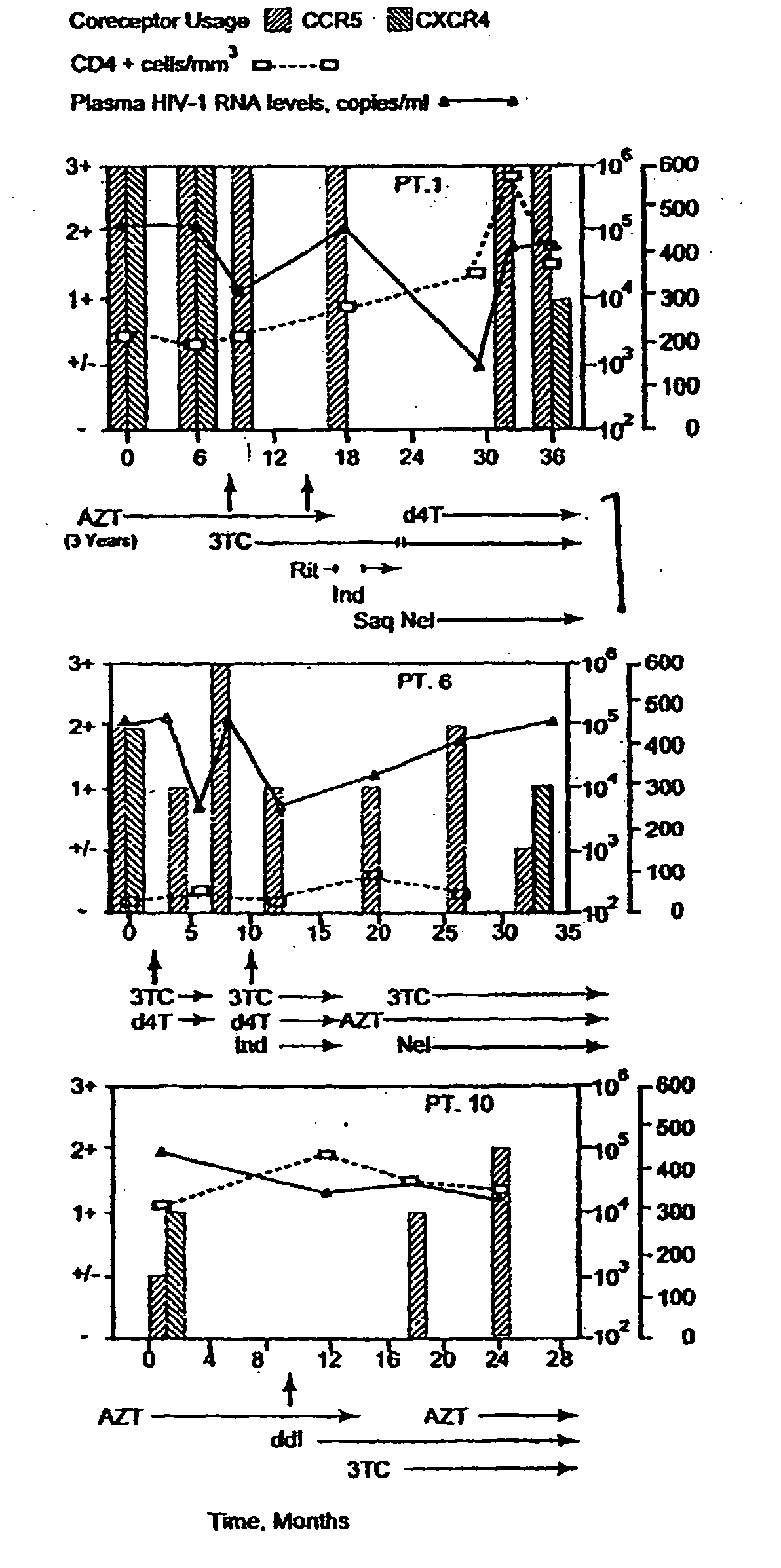
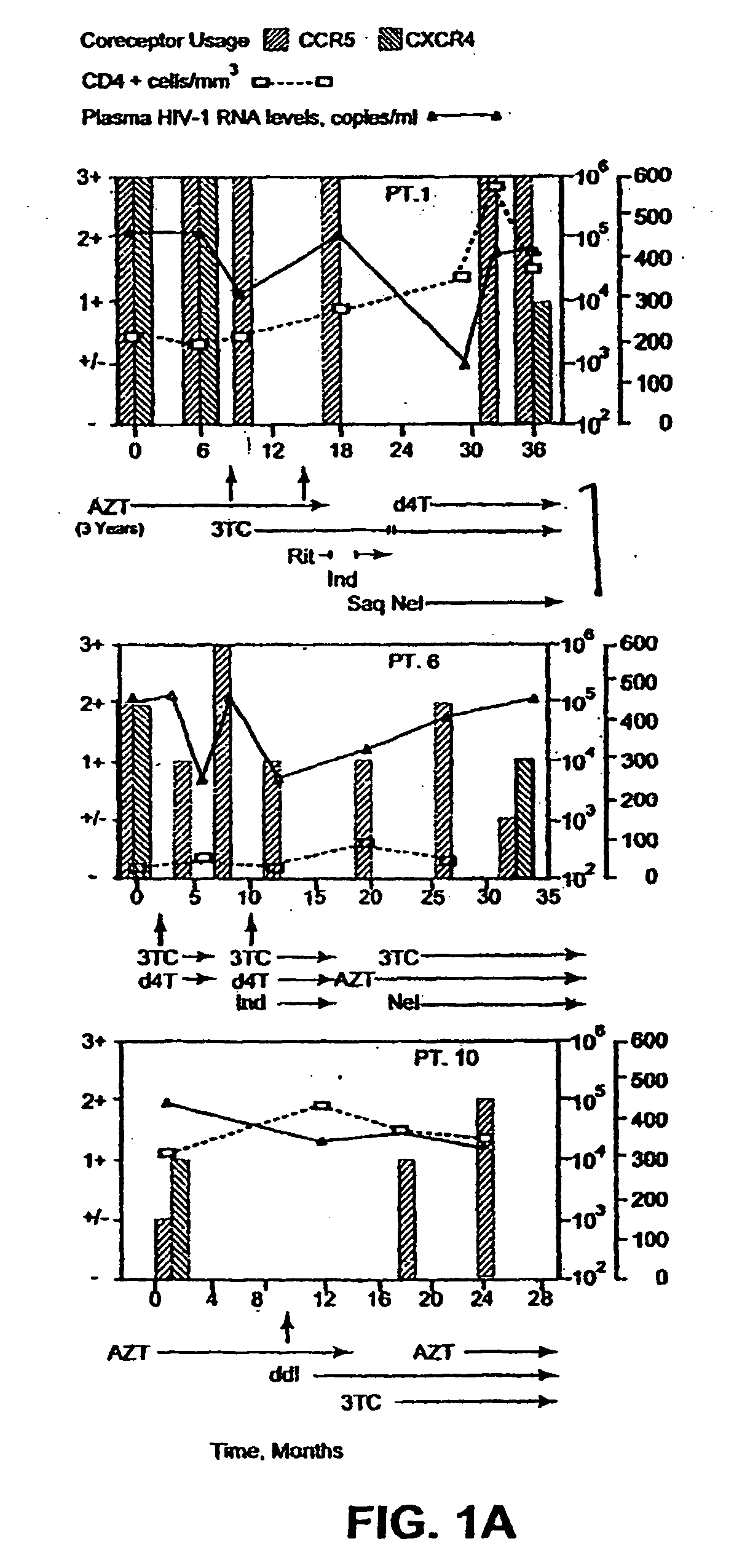
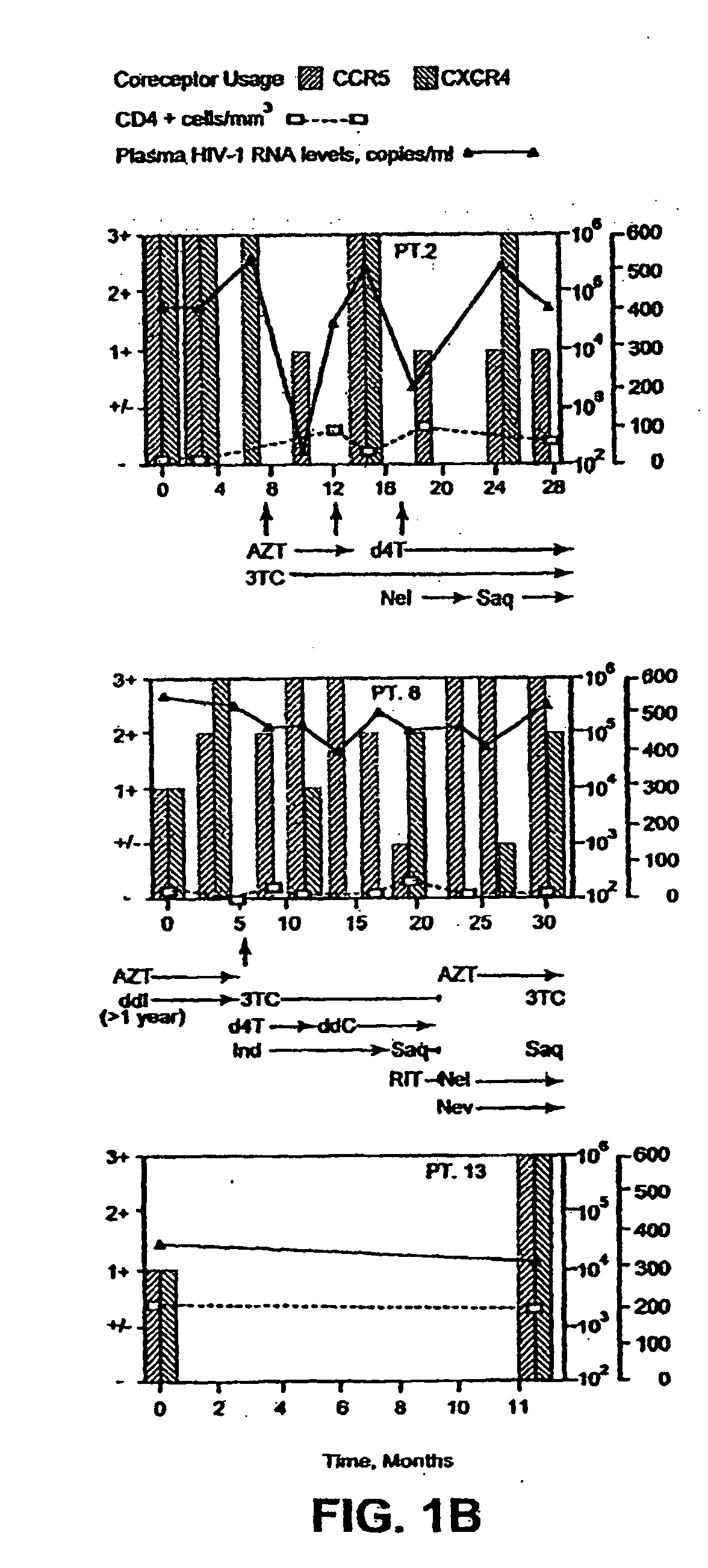
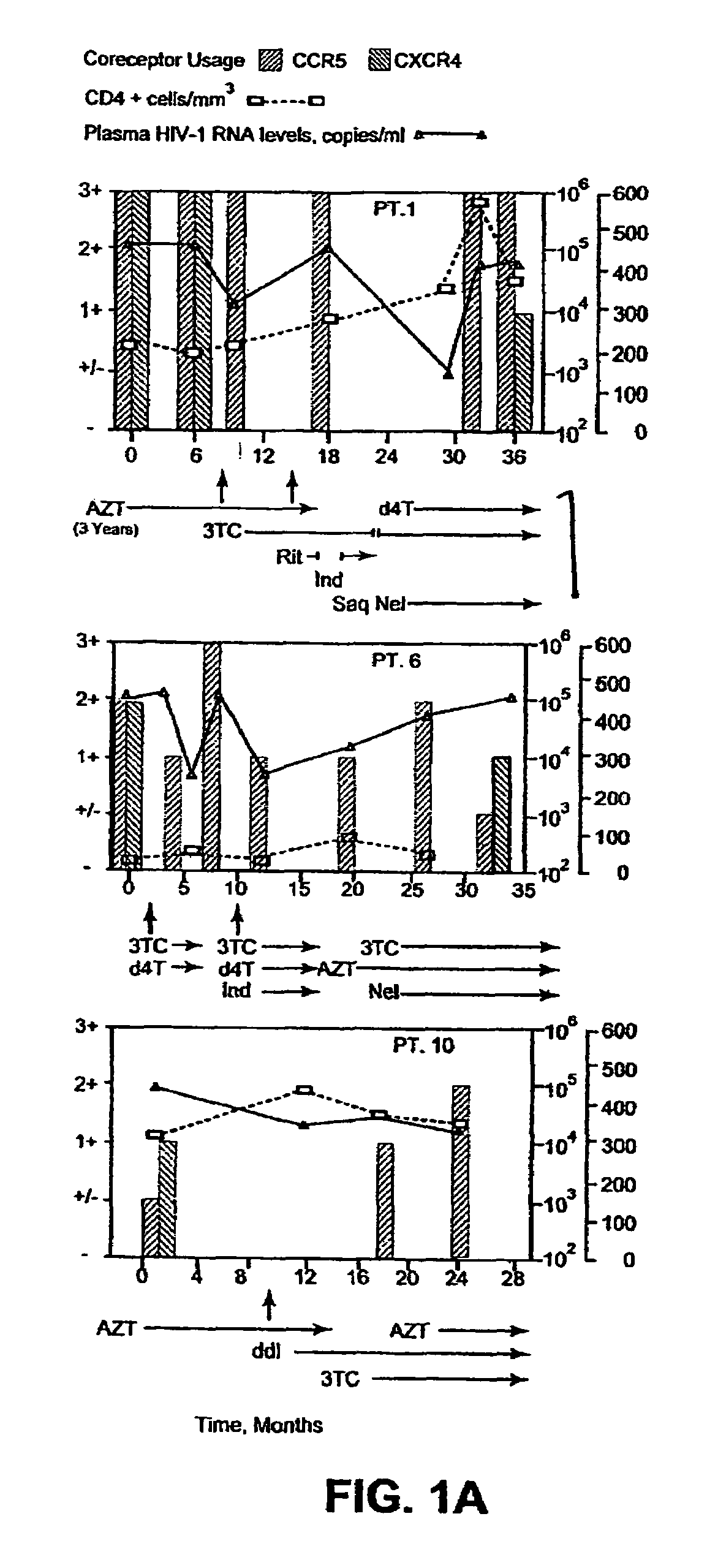
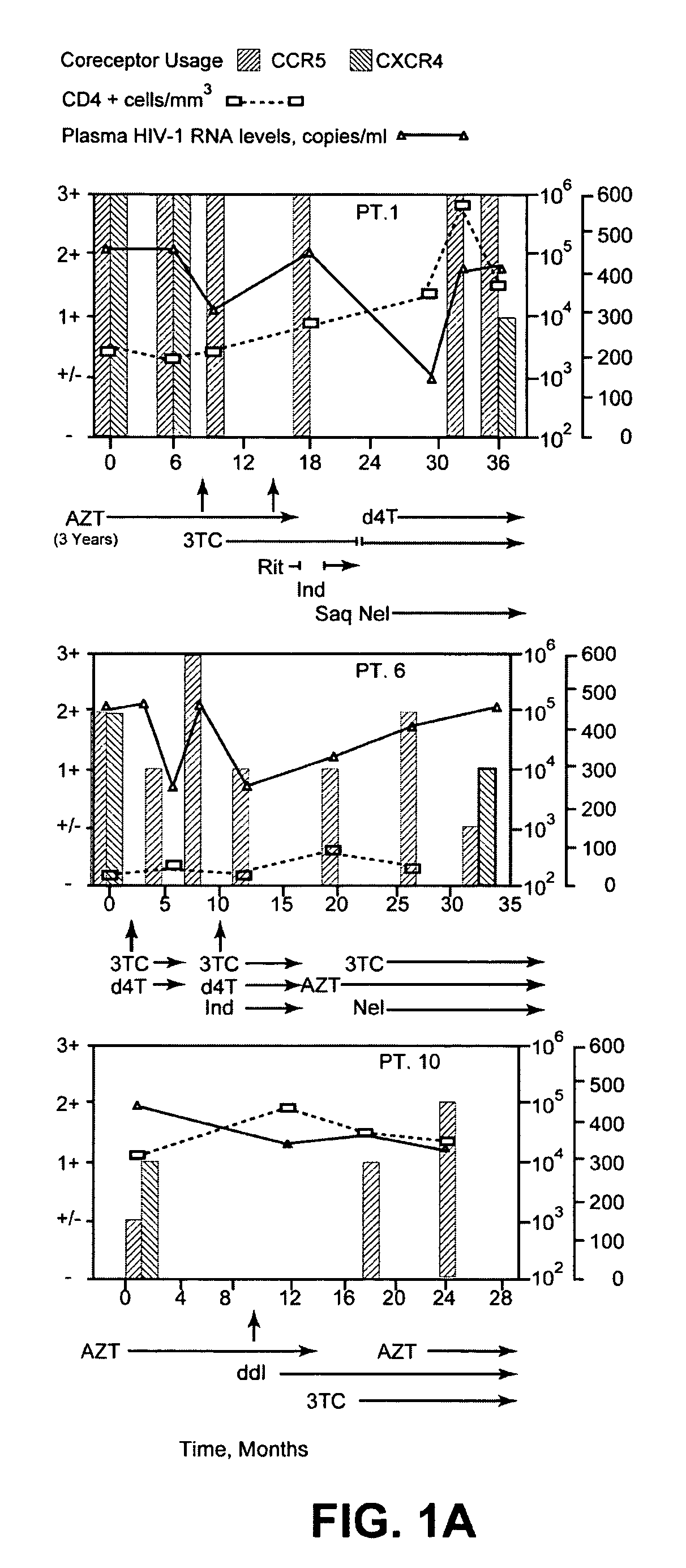
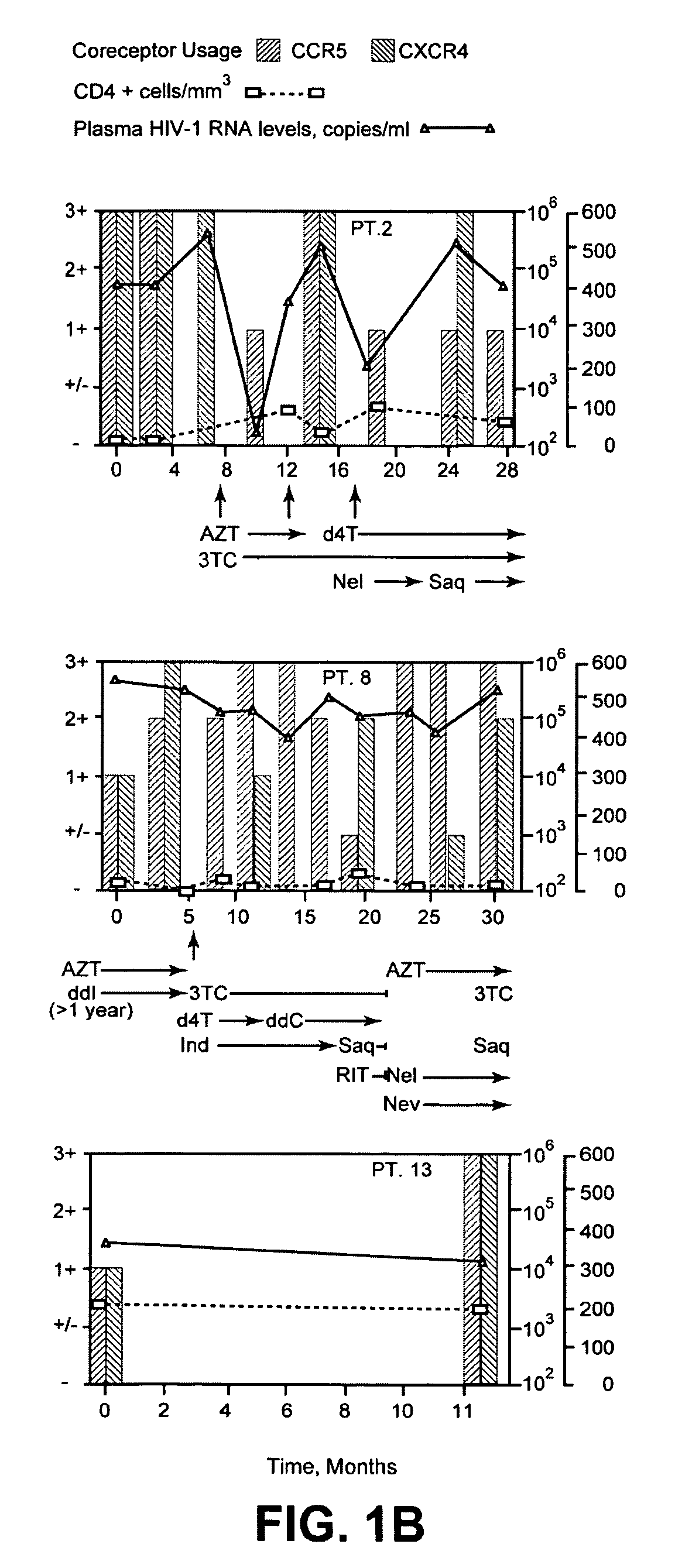
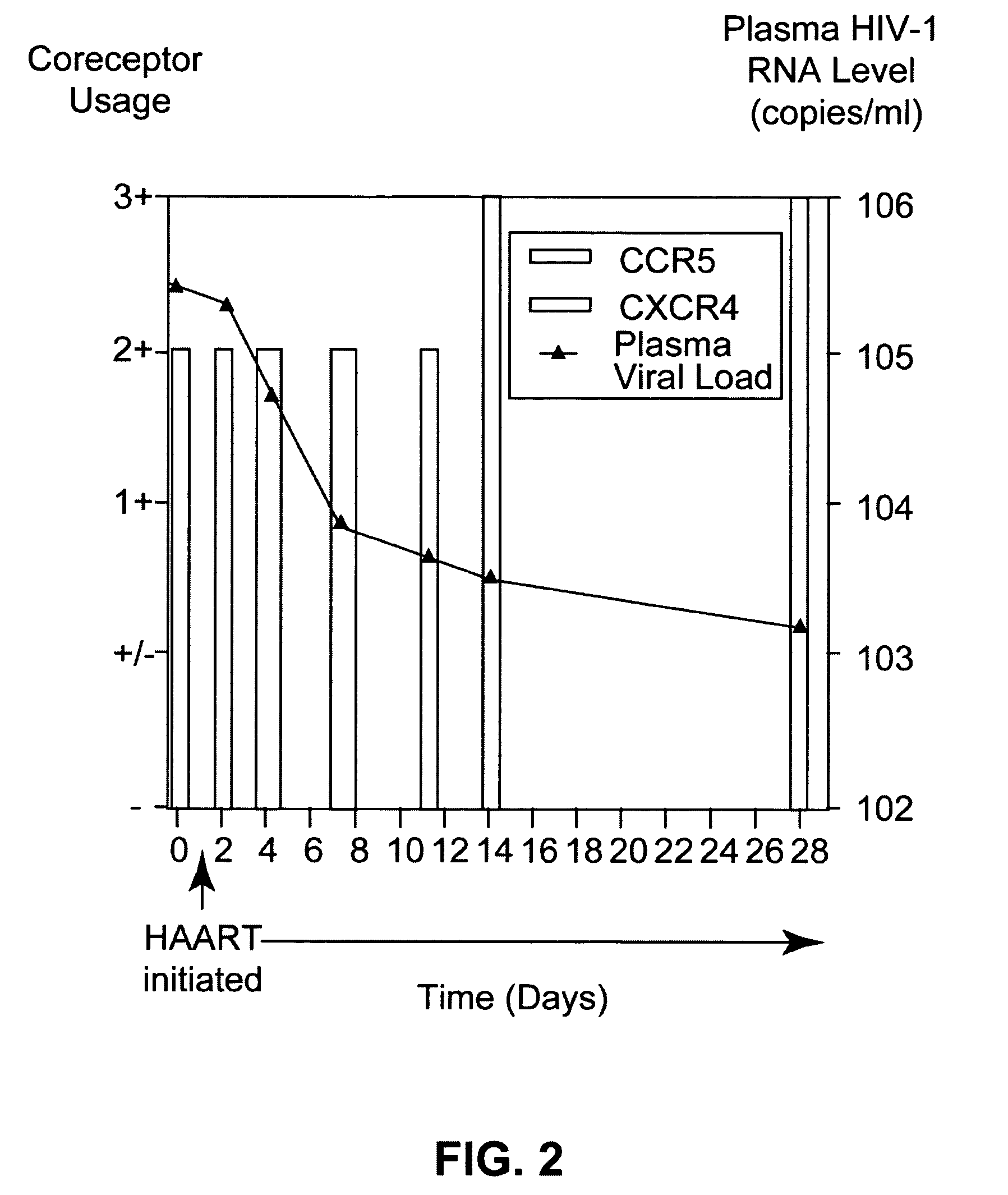
Analysis of HIV-1 coreceptor use in the clinical care of HIV-1-infected patients
InactiveUS6727060B2Accurate predictionMore effectivenessMicrobiological testing/measurementArtificial cell constructsImmunodeficiency virusHIV positives
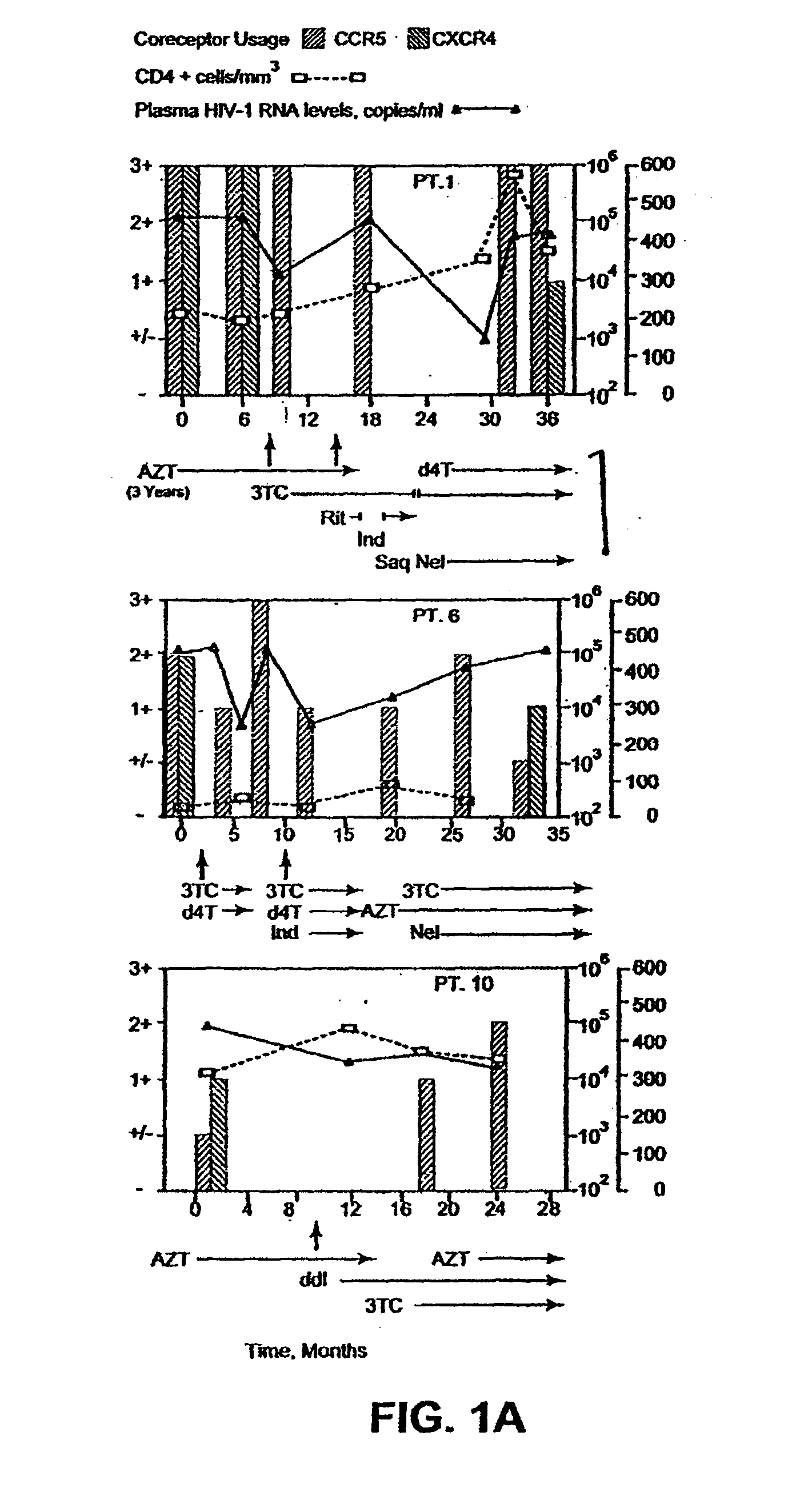
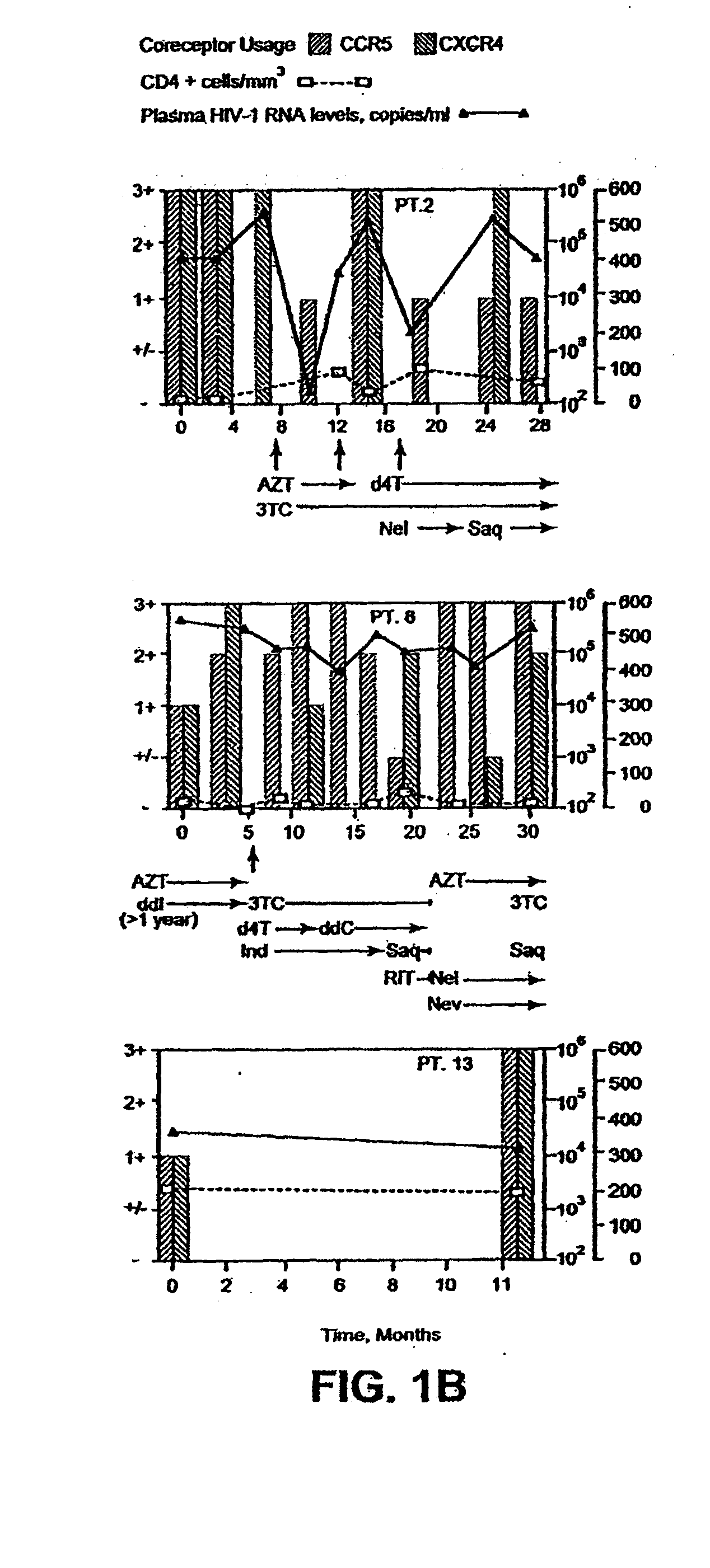
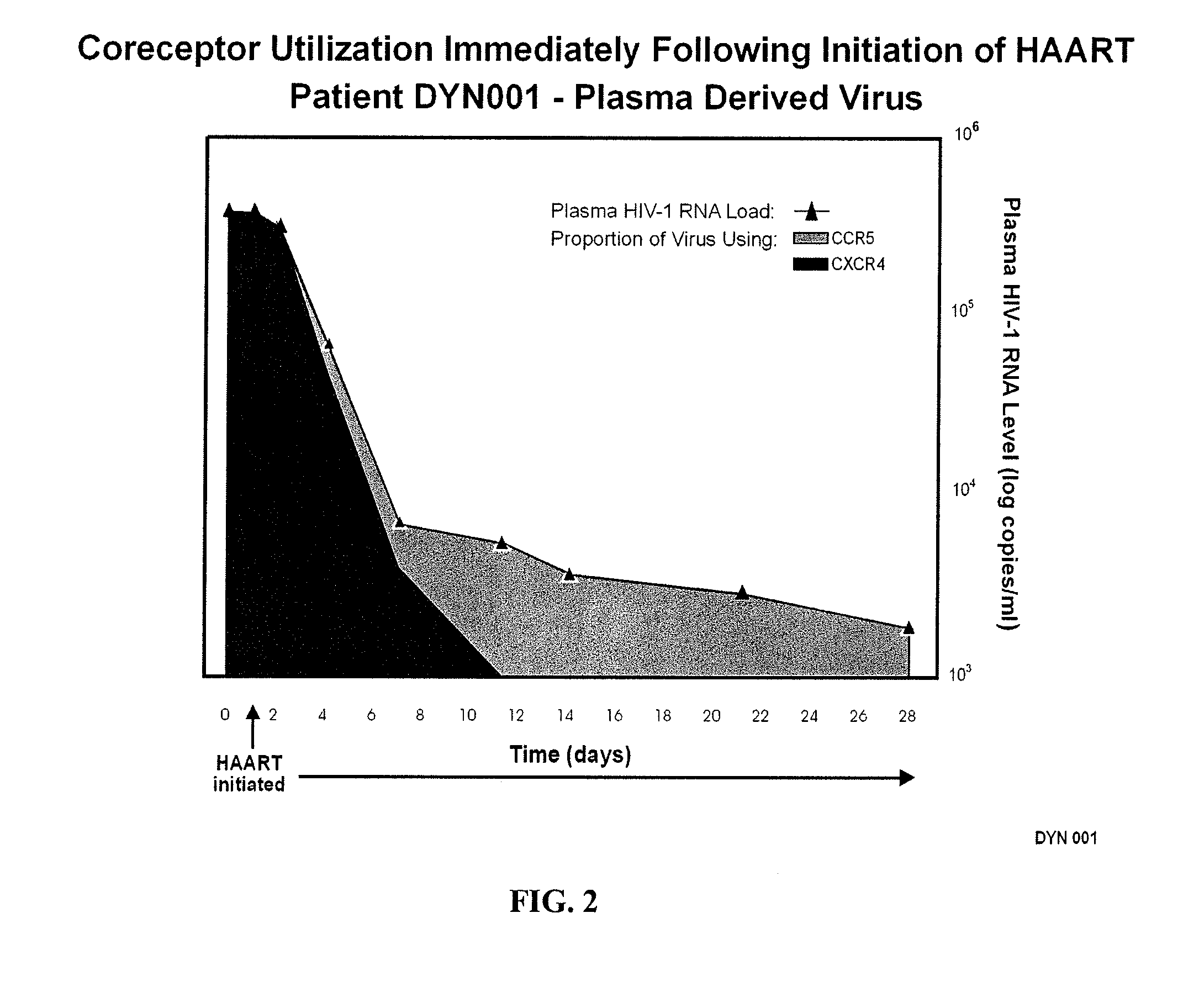
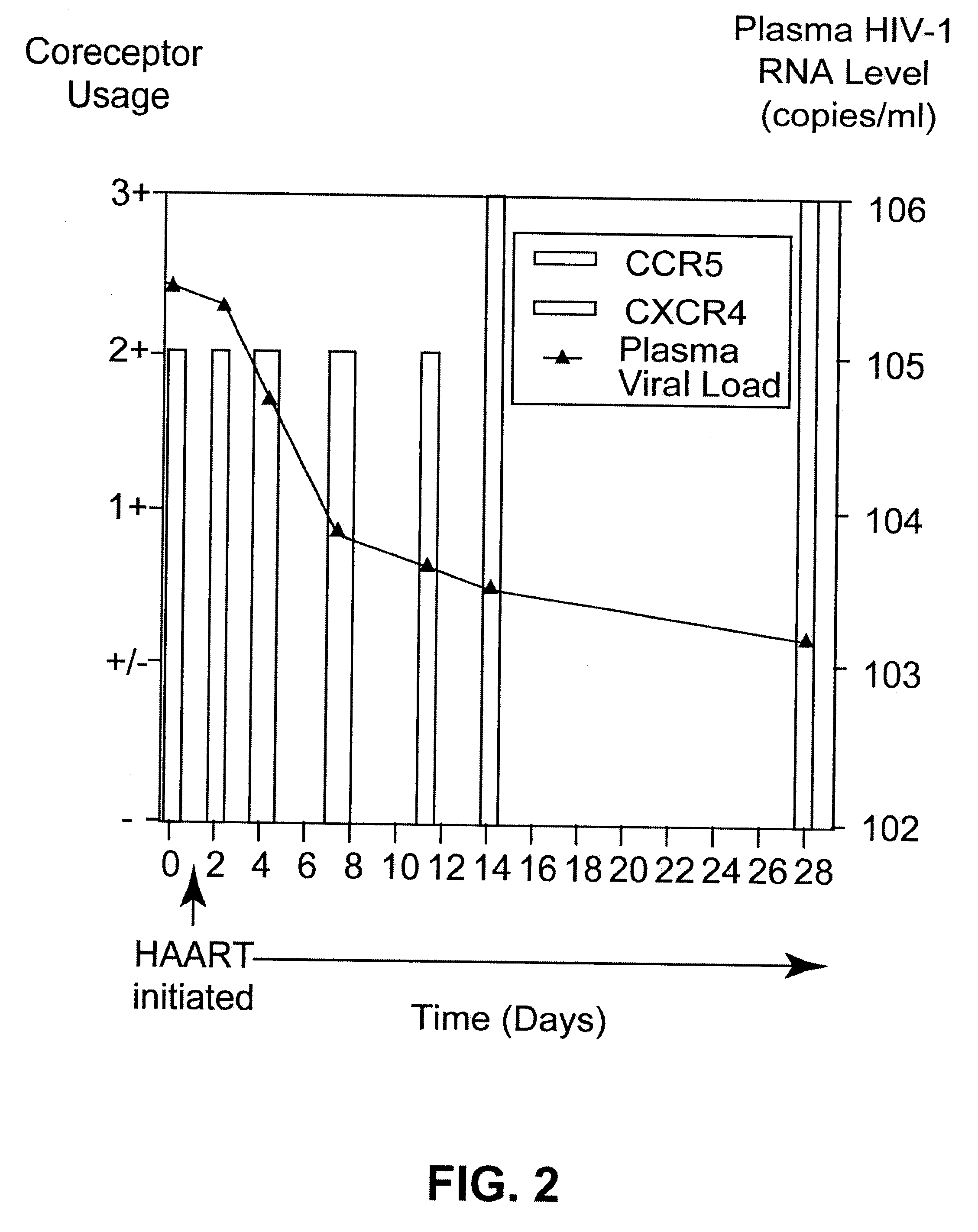
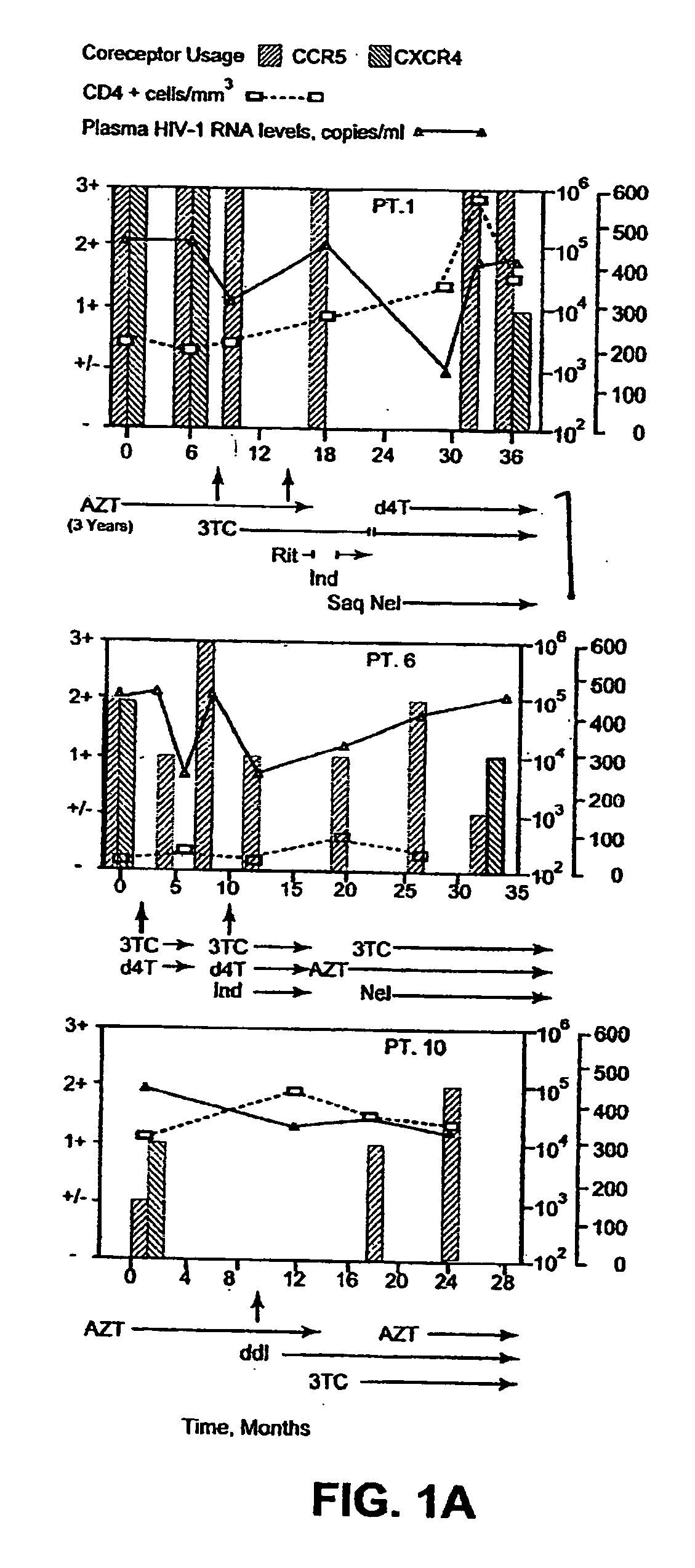
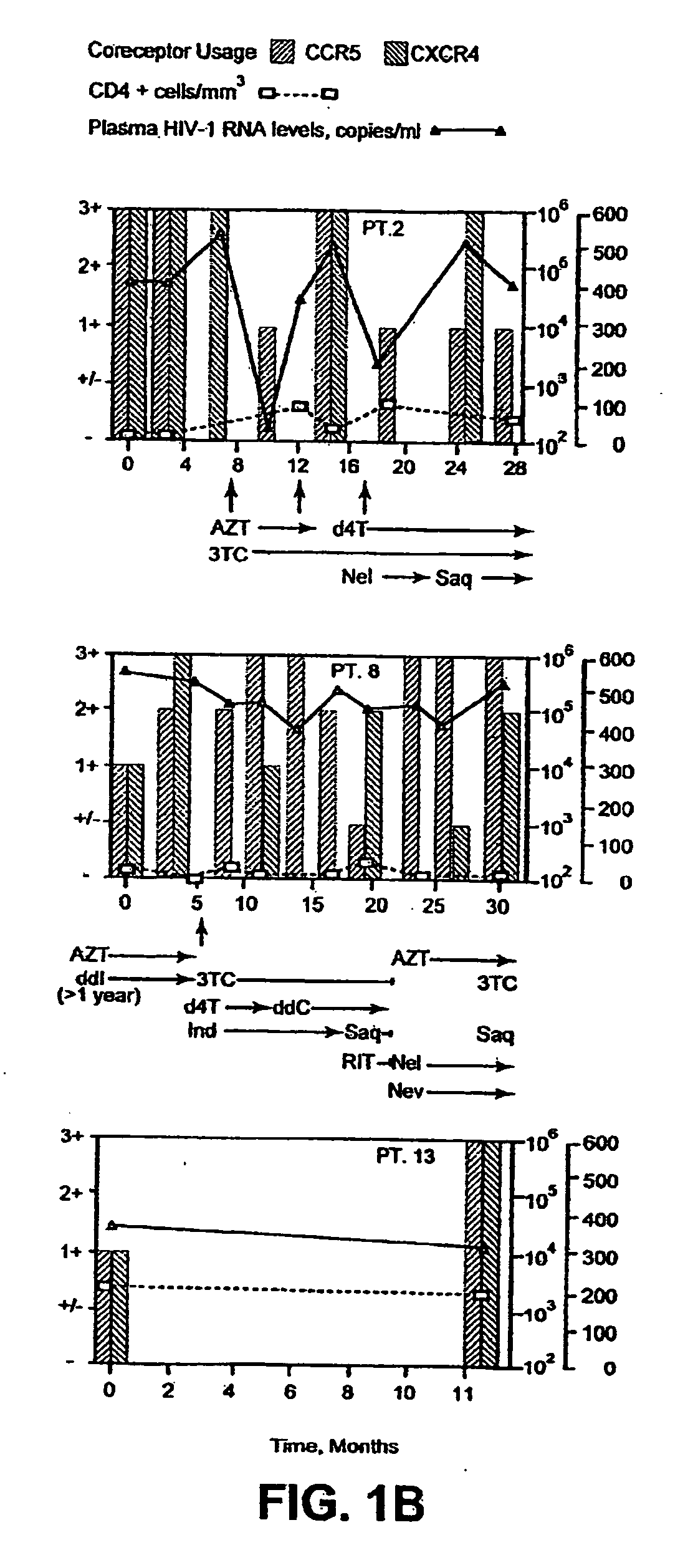
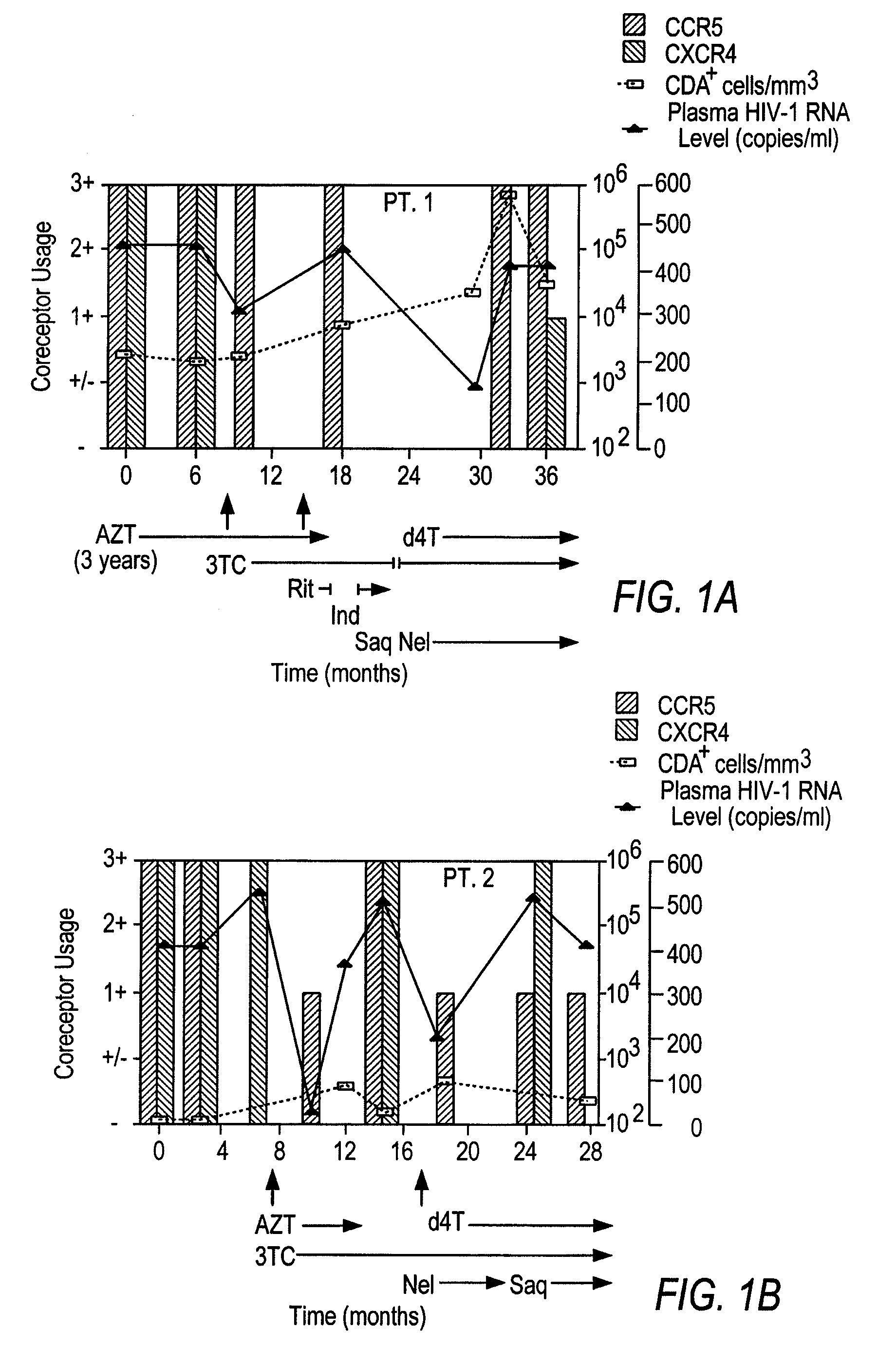
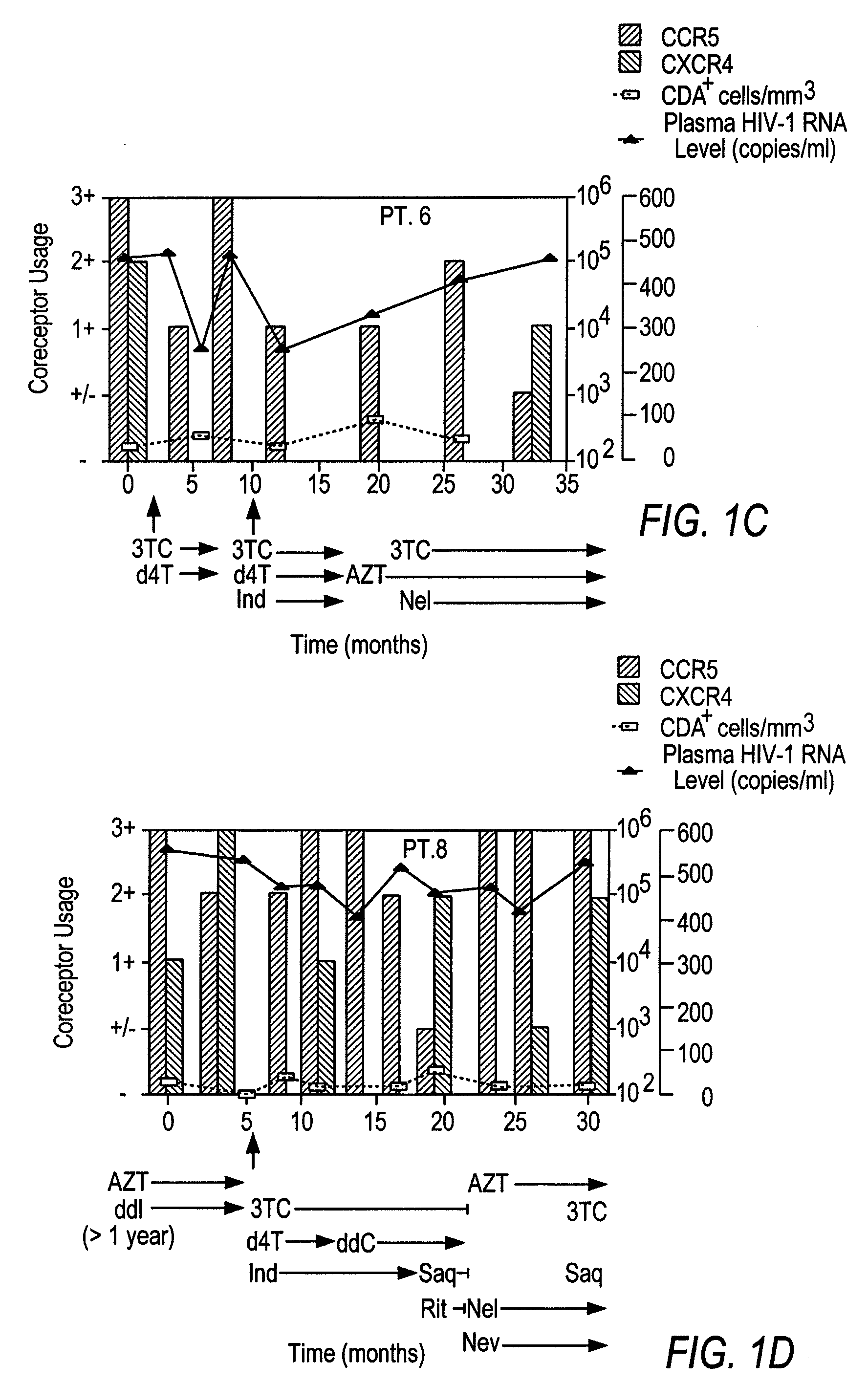
A change in viral tropism occurs in many HIV positive individuals over time and can be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus can be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CXCR4 specific strains. The diagnostic methods can be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time.
Owner:HEALTH RES INC
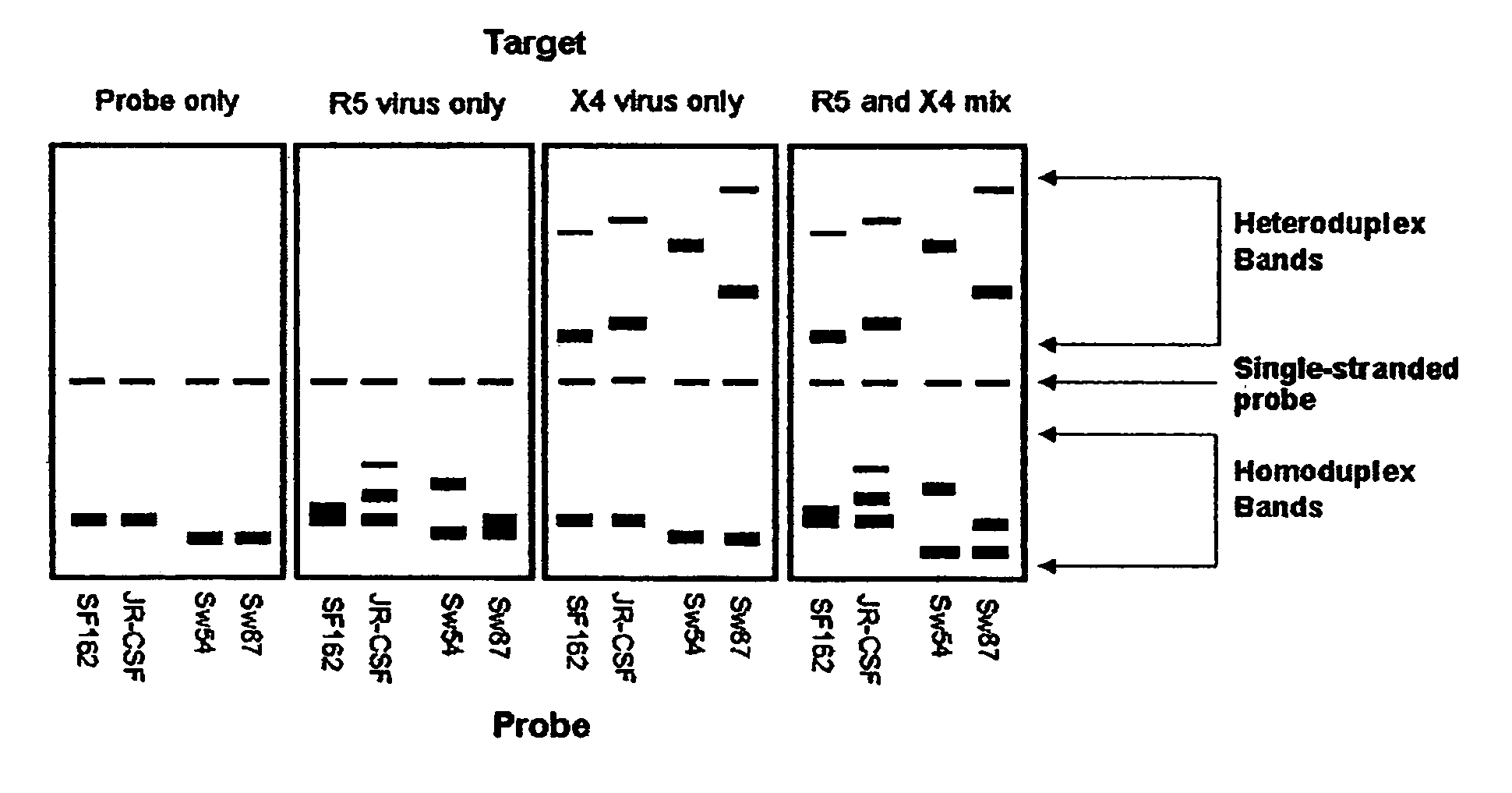
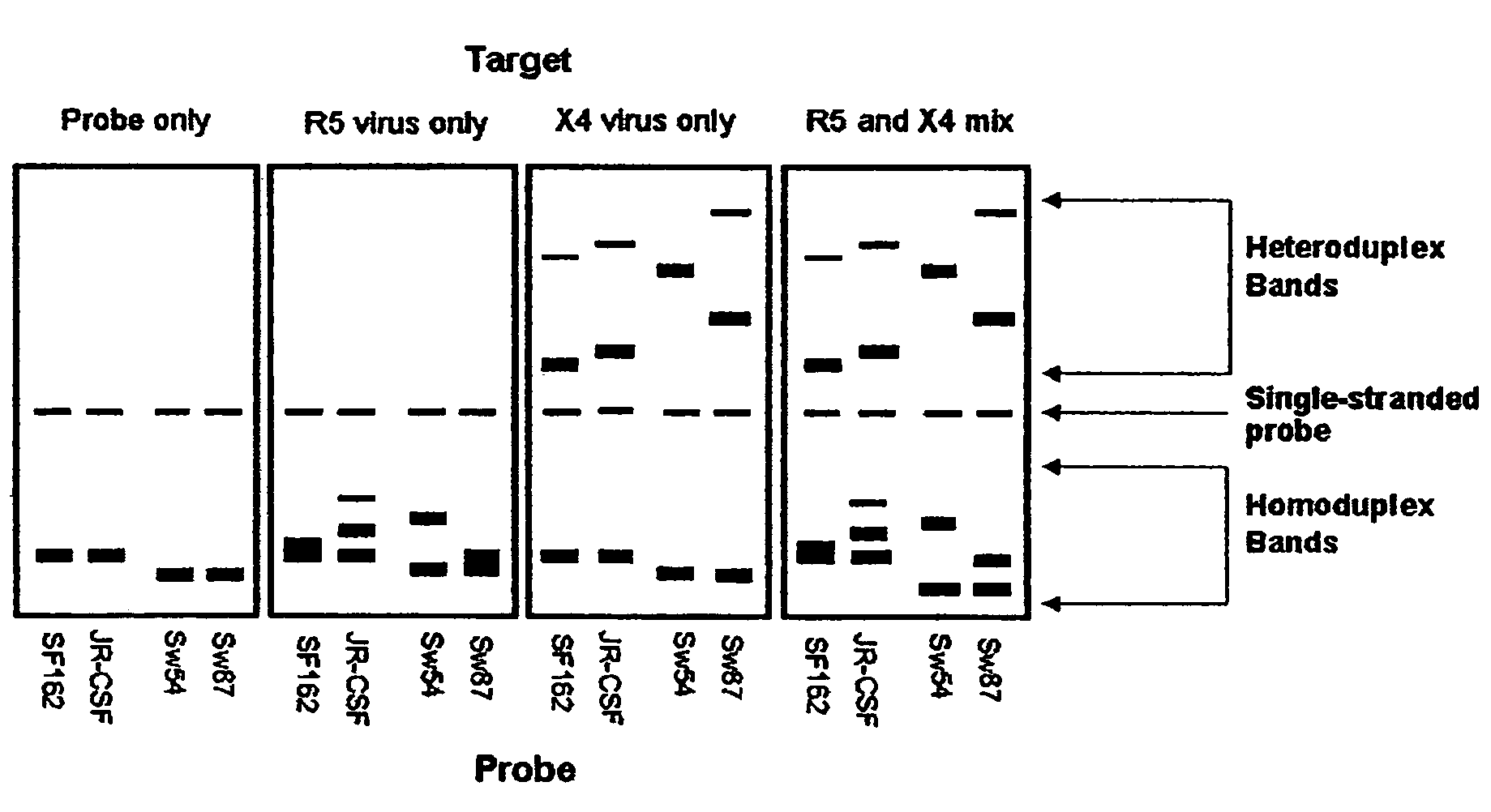
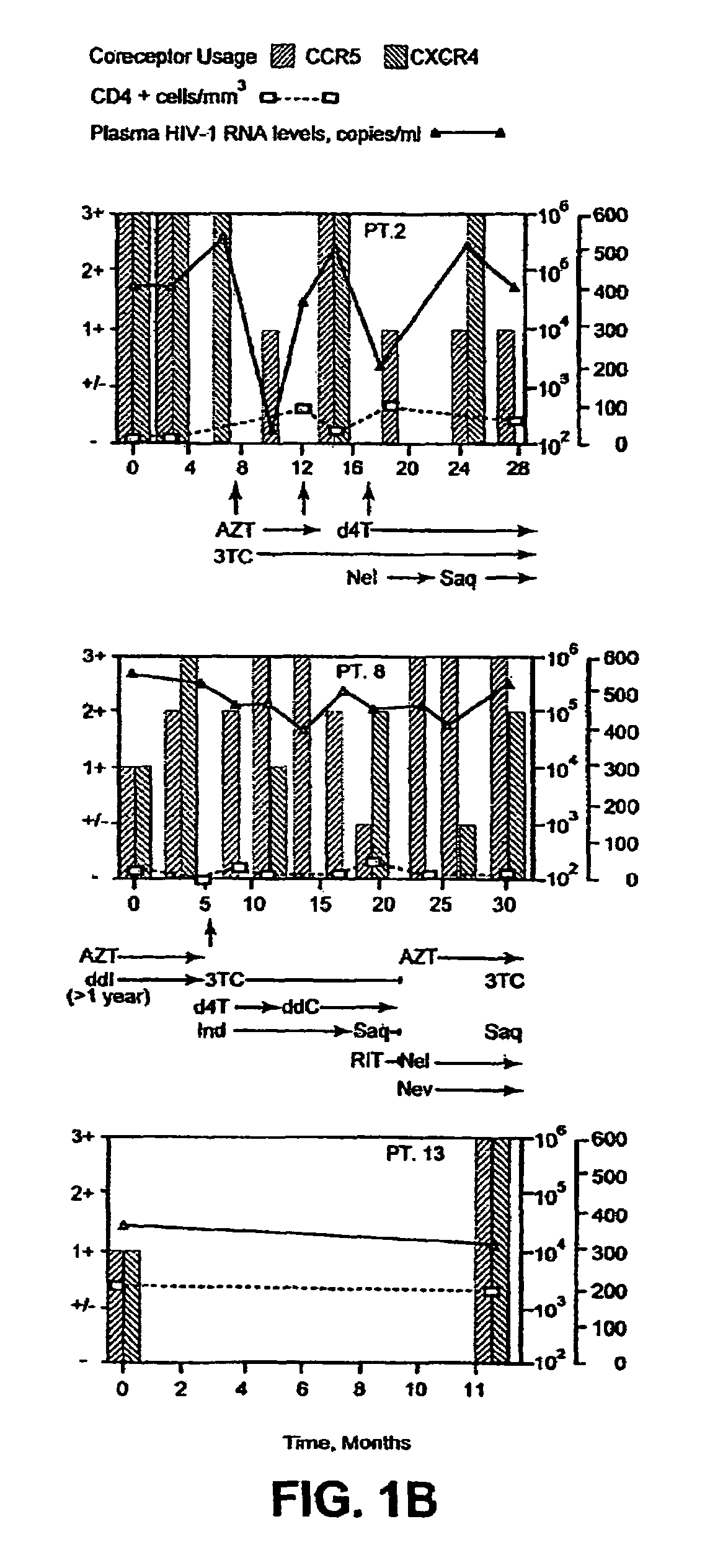
Heteroduplex tracking assay
InactiveUS20060194227A1Accurately predict disease prognosis over timeGood treatment effectMicrobiological testing/measurementVertebrate cellsHeterologousHeteroduplex
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
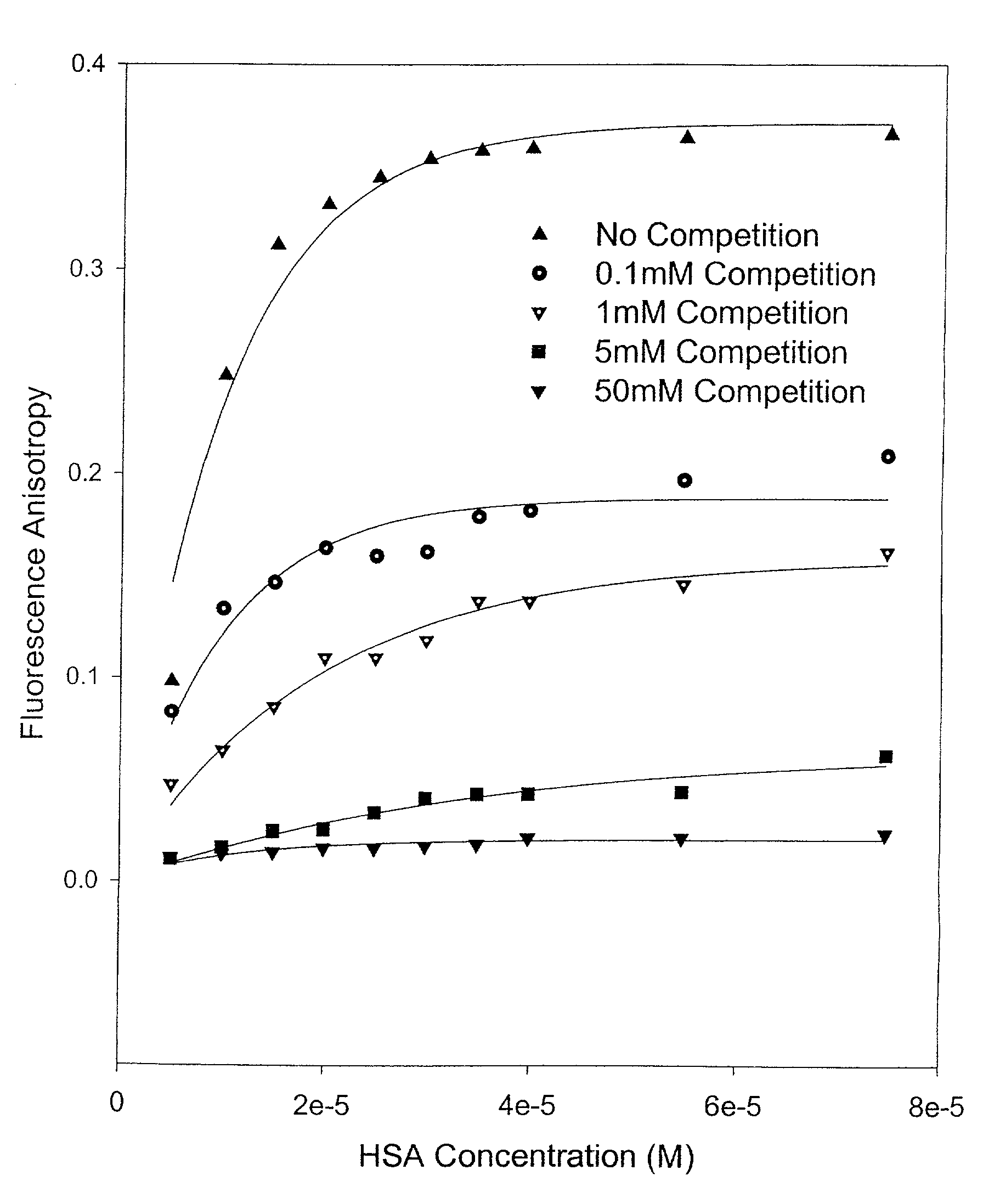
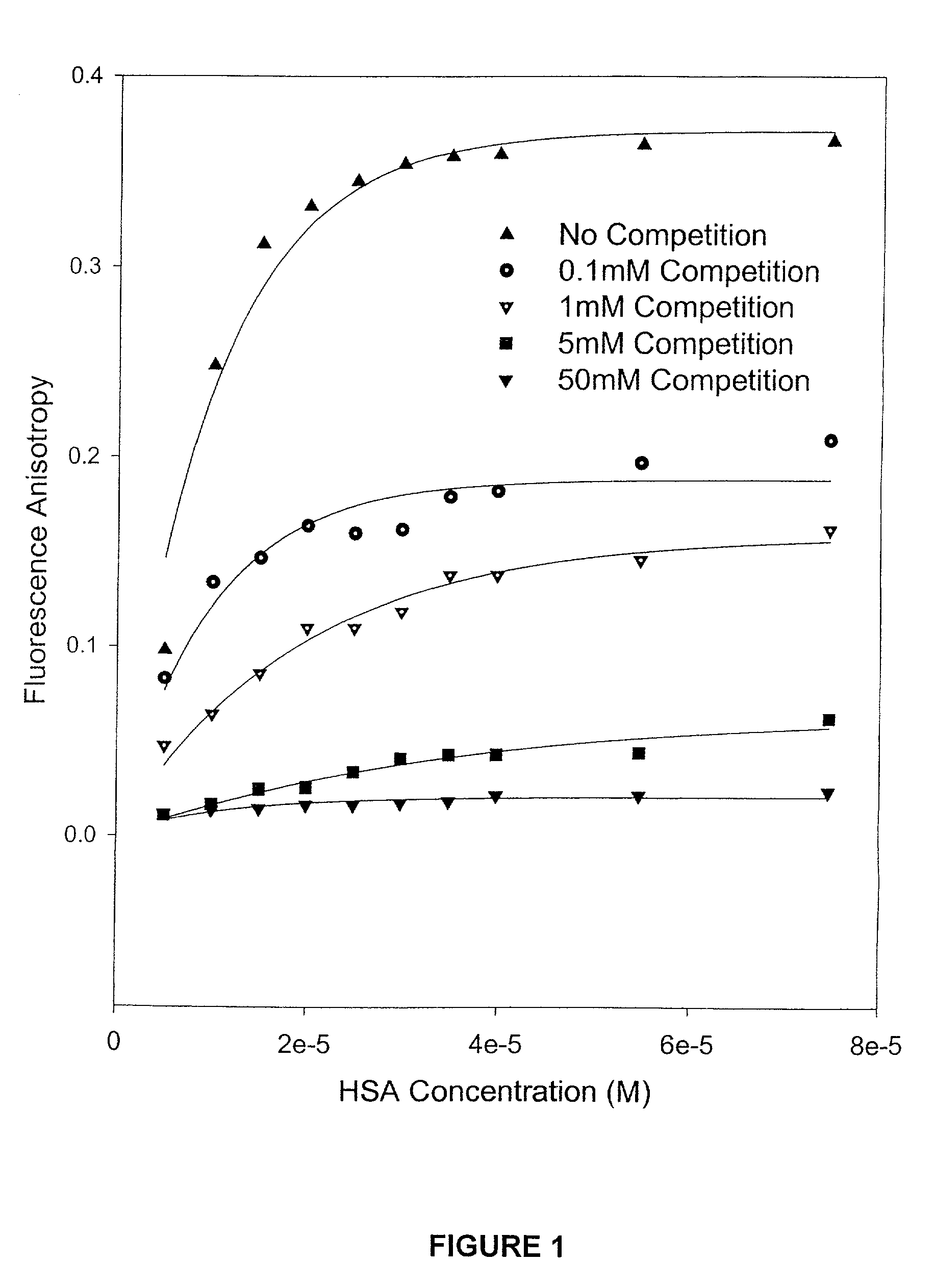
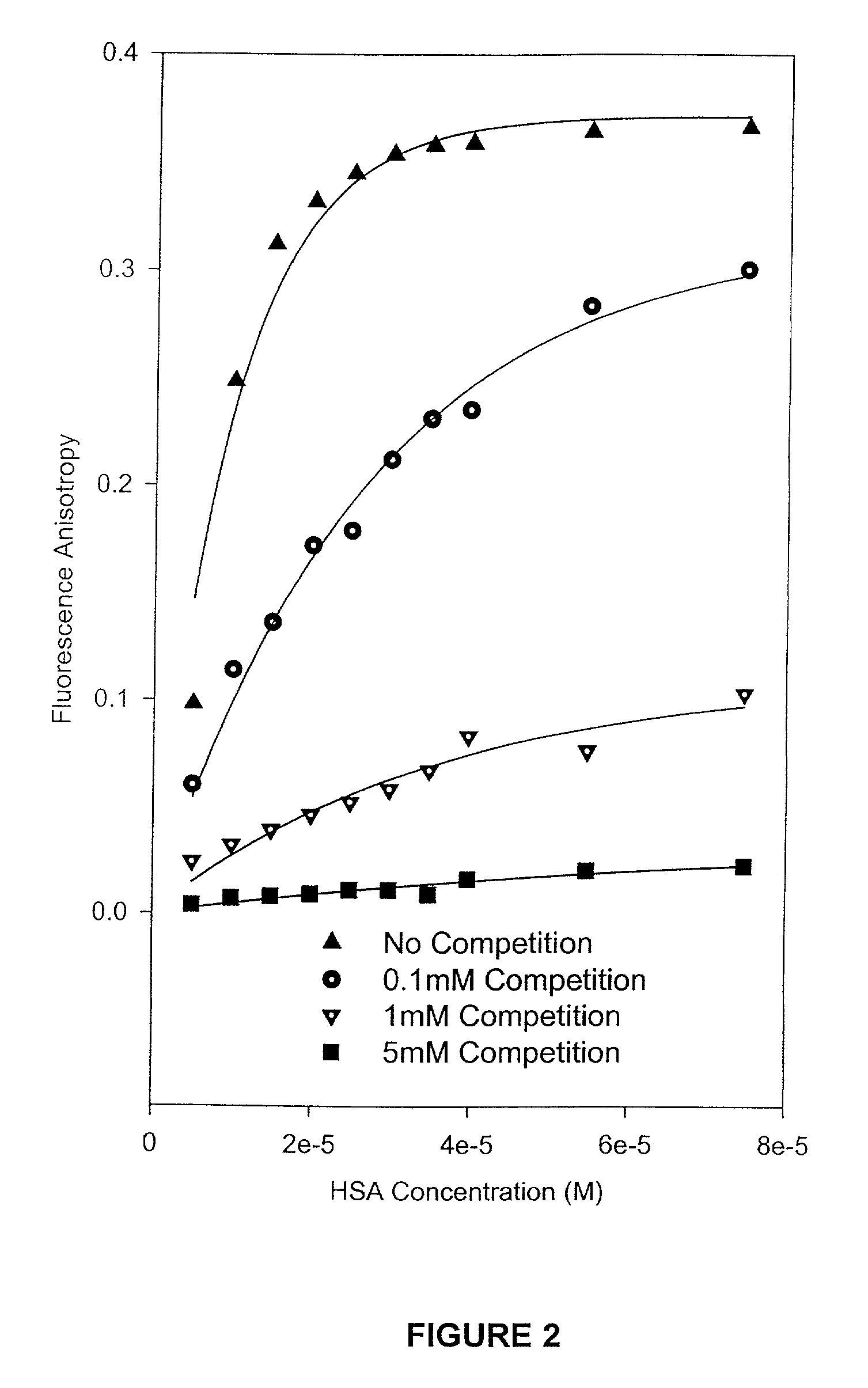
Methods and compositions for optimizing blood and tissue stability of camptothecin and other albumin-binding therapeutic compounds
InactiveUS20100240602A1Improve stabilityHigh affinitySalicyclic acid active ingredientsBiocideNitrocamptothecinHIV positives
The present invention provides methods and formulations for optimizing the anti-cancer and anti-HIV activities of a camptothecin drug, including camptothecin and its related analogs including 9-aminocamptothecin and 9-nitrocamptothecin. The invention involves methodologies and formulations that limit human serum albumin-mediated reduction of the anti-cancer and anti-HIV effects of the camptothecins, and the methods and formulations provide combination therapies in which binding of the camptothecin agent to human serum albumin can be modulated by the administration of a competing agent such as ibuprofen, clofibrate or clofibric acid that also binds human serum albumin. Reduced camptothecin drug binding to human serum albumin can result in elevated camptothecin free drug levels and thus improve the effectiveness of treatment regimens involving these drugs. Further agents such as methotrexate and AZT can also be used in cancer and HIV-positive patients employing camptothecin drugs.
Owner:BURKE THOMAS G +1
Composition of anti-HIV drugs and anti-cortisol compounds and method for decreasing the side effects of anti-HIV drugs in a human
InactiveUS20050085464A1Elevated triglycerideElevated cholesterolBiocideCarbohydrate active ingredientsProcaineSide effect
The present invention is based, in part, upon the discovery that the use of an anti-HIV drug in combination with at least one cortisol blocker such as procaine, reduces the side effects associated with anti-HIV drugs. The invention also relates to a method of treating the high cortisol catabolic effects of diseases such as AIDS in the HIV positive population and those with AIDS related complexes by the administration of a cortisol blocker. The present invention also discloses a composition comprising an anti-HIV drug and cortisol blocker. More specifically, the present invention relates to a cortisol blocking composition which comprises procaine, ascorbic acid and zinc heptahydrate.
Owner:SAMARITAN PHARMA
Heteroduplex tracking assay
Owner:HEALTH RES INC
HIV positive serum surrogate
Substitute of HIV positive blood serum is cross-linking object obtained from cross-linking reaction between unhuman anti HIV antibody or its treating fluid and normal person immunoglobulin or its treating fluid. Essence of the invention is to combine unhuman anti HIV antibody activity (i.e. activity reacting to HIV antigen) with antigen activity of normal person immunoglobulin (i.e. activity reacting to the conjugate of anti abzyme of human immunoglobulin) so as to eliminate risk of containing pathogenesis factor possibly in blood serum (or blood plasma), which is positive in human anti antibody of pathogenesis factor, but possess reactivity on blood serum, which is positive in anti antibody of pathogenesis factor. Since the substitute is from unhuman anti HIV antibody and normal person immunoglobulin, thus it is essential to prevent risk infected by positive serum potentially.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST
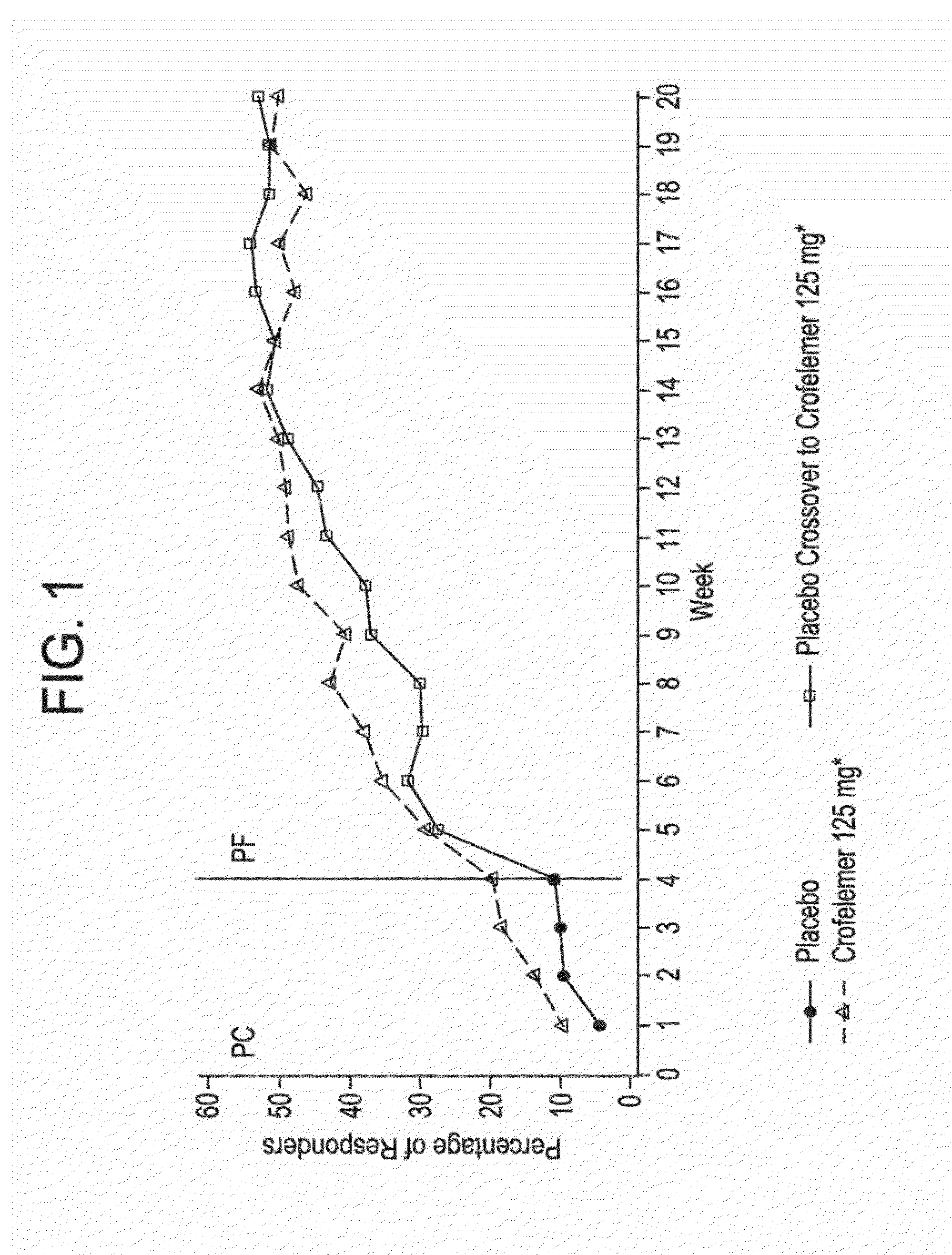
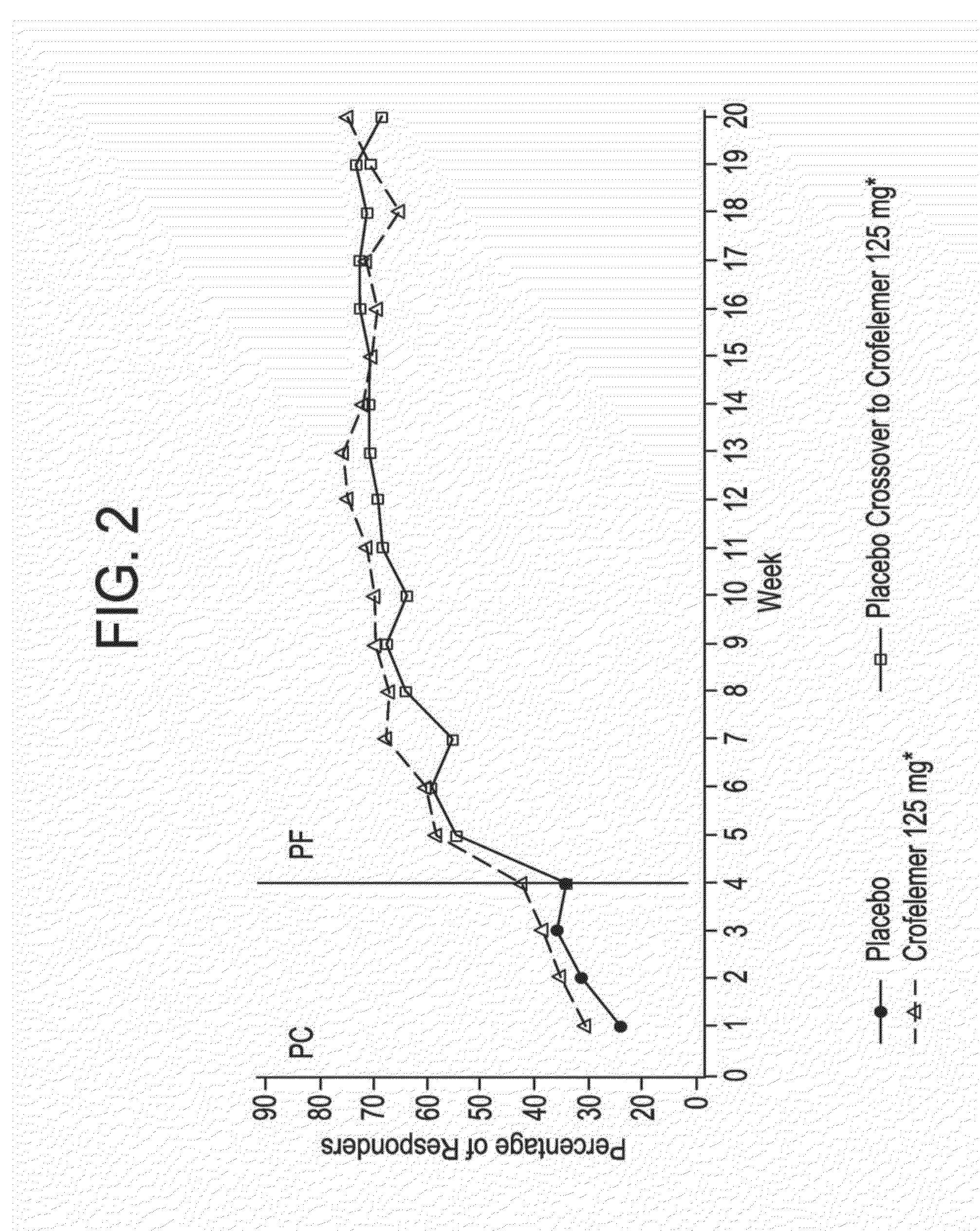
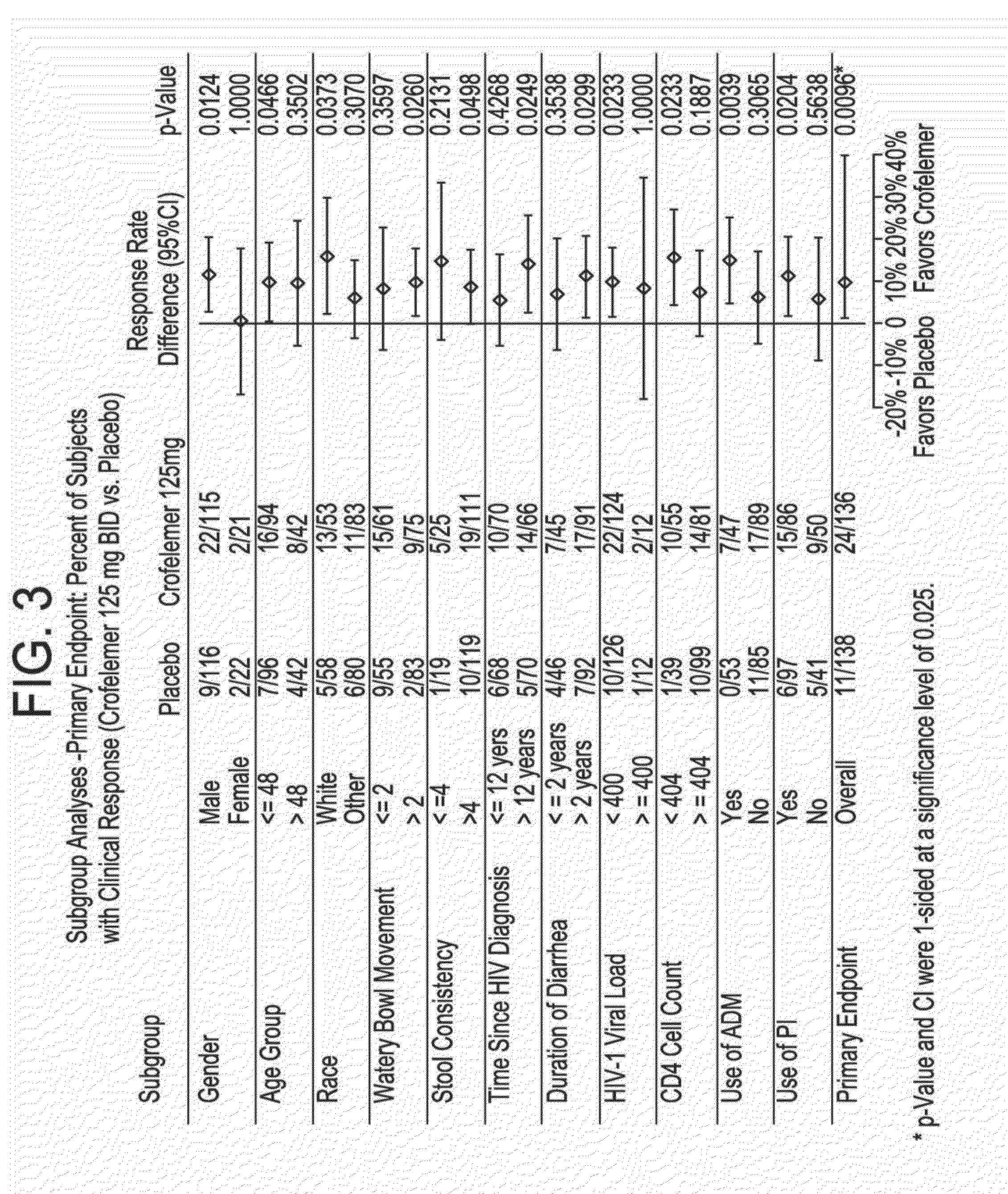
Methods and compositions for treating hiv-associated diarrhea
InactiveUS20140163096A1Not cause deterioration of immune statusBiocideDigestive systemDrug-drug interactionAntiretroviral therapy
Provided herein are methods for treating HIV-associated or highly active antiretroviral therapy (HAART)-associated diarrhea in an HIV positive subject by administering a composition comprising crofelemer to the subject wherein the composition has minimal drug-drug interactions with at least one other compound concurrently administered to the subject to treat an HIV infection. Also provided are methods for treating HIV-associated or highly active antiretroviral therapy (HAART)-associated diarrhea in an HIV positive subject by administering a composition comprising crofelemer to the subject, wherein the composition does not significantly inhibit the activity of at least one other compound concurrently administered to the subject to treat an HIV infection.
Owner:NAPO PHARMA INC
Detection of drug-resistant human immunodeficiency virus
InactiveUS7232657B2Sugar derivativesMicrobiological testing/measurementImmunodeficiency virusMedicine
Owner:UNIV OF MASSACHUSETTS
Heteroduplex tracking assay
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry soon after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
Owner:HEALTH RES INC
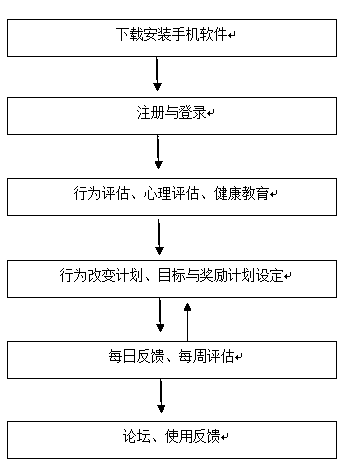
Mobile phone software for behavior intervention aiming at MSM
The invention relates to mobile phone software for behavior intervention aiming at MSM. The mobile phone software for MSM behavior intervention comprises modules of health assessment, health education, behavior change planning, forum and the like. Behavior assessment aiming at MSM can be performed, a pertinent behavior change plan and a personal incentive plan can be formulated, thus daily behavior feedback and weekly behavior assessment can be performed, education of knowledge about AIDS can be performed, sexual behaviors, drug abuse, medication compliance for antiviral therapy of HIV positive patients and the like can be intervened, and thus the purposes of preventing AIDS virus infection and promoting physical and psychological health can be achieved.
Owner:严谨
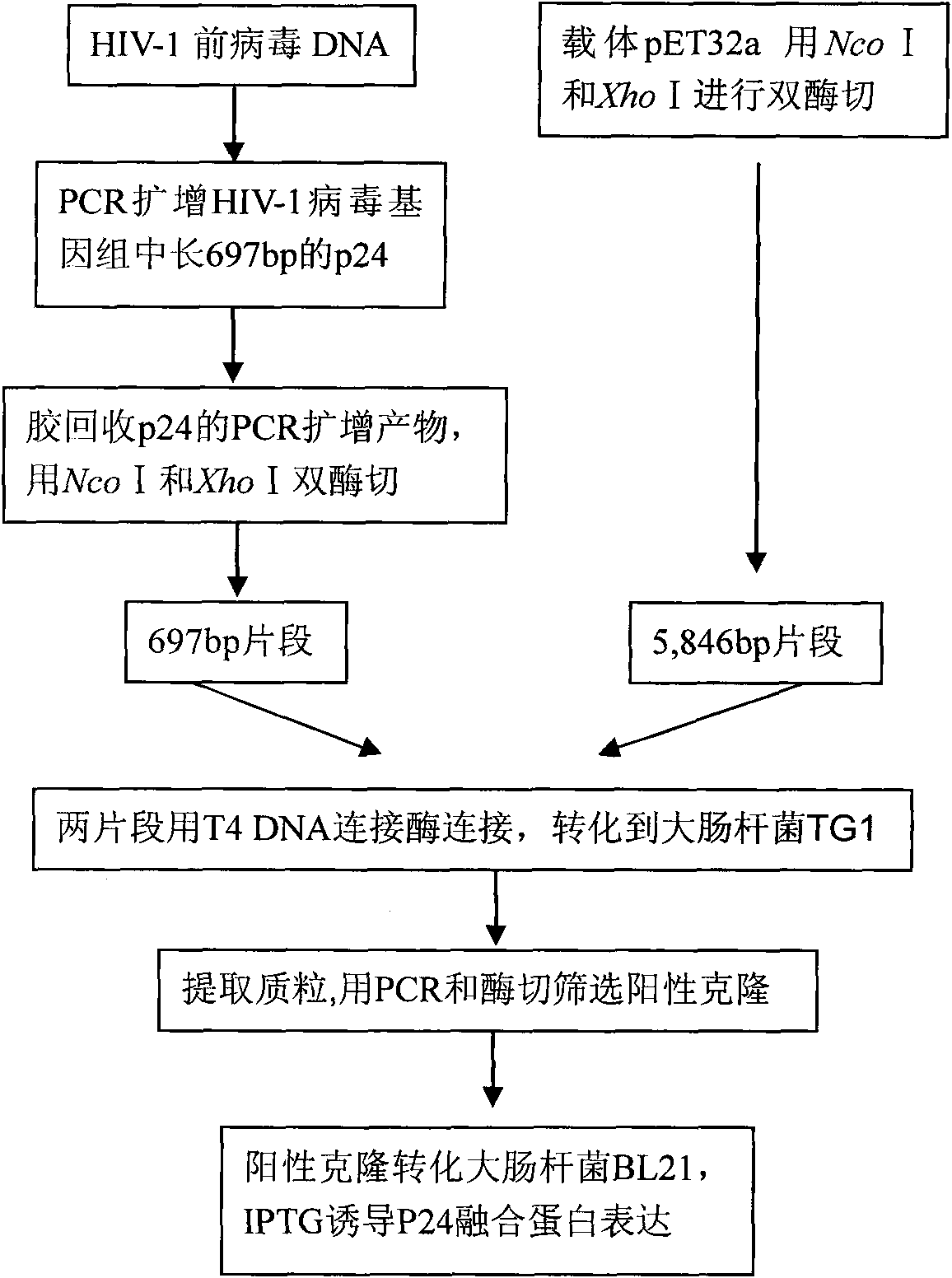
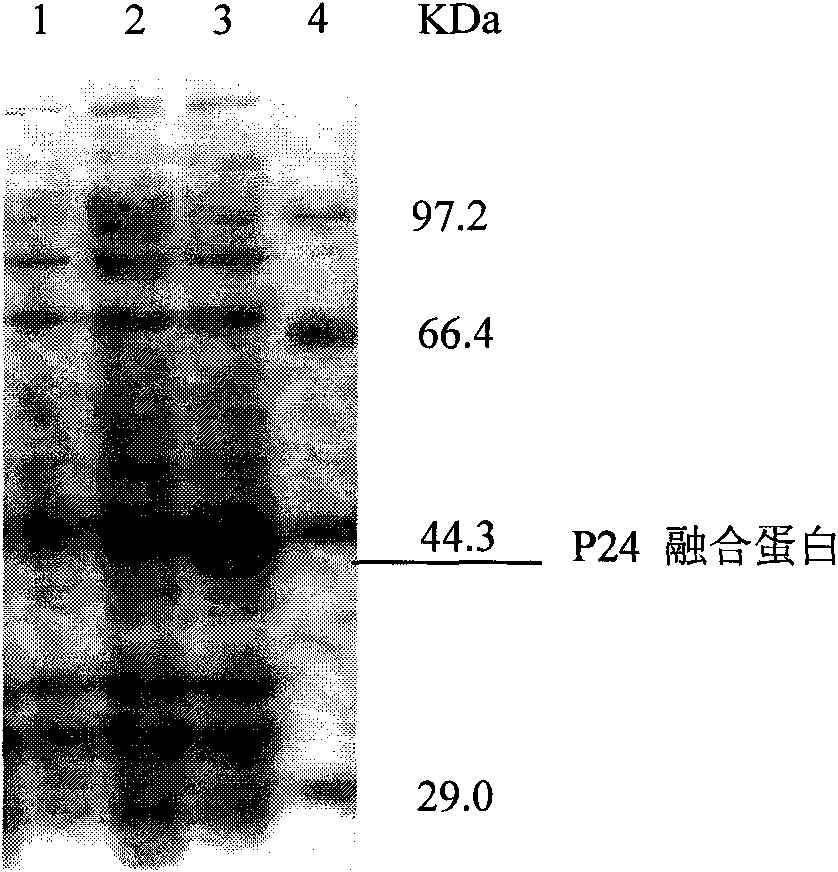
Monoclonal antibody hybridoma cell of HIV P24 and application
InactiveCN101671655AIncreased sensitivityImprove featuresTissue cultureMaterial analysisSpecific detectionHIV positives
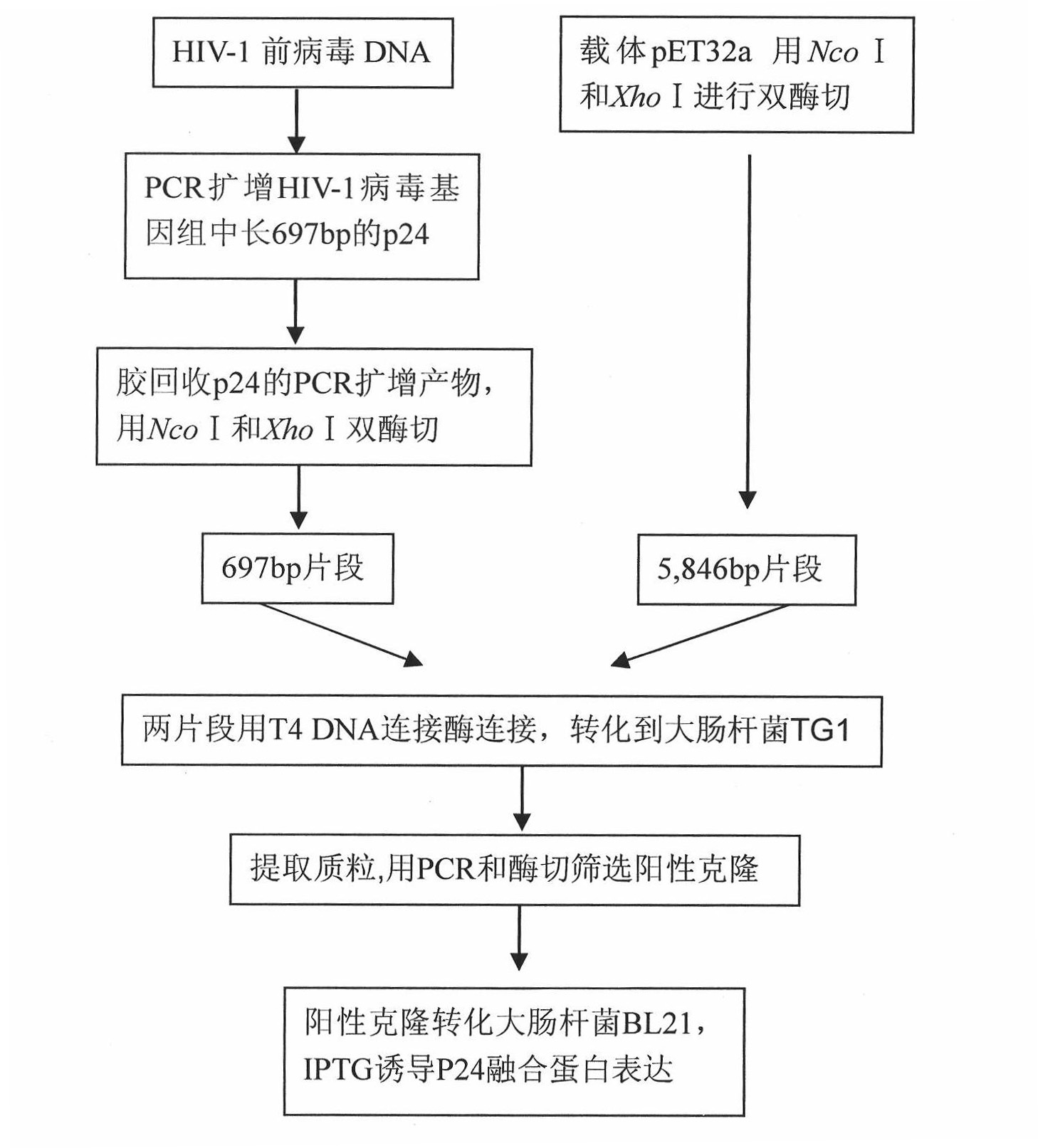
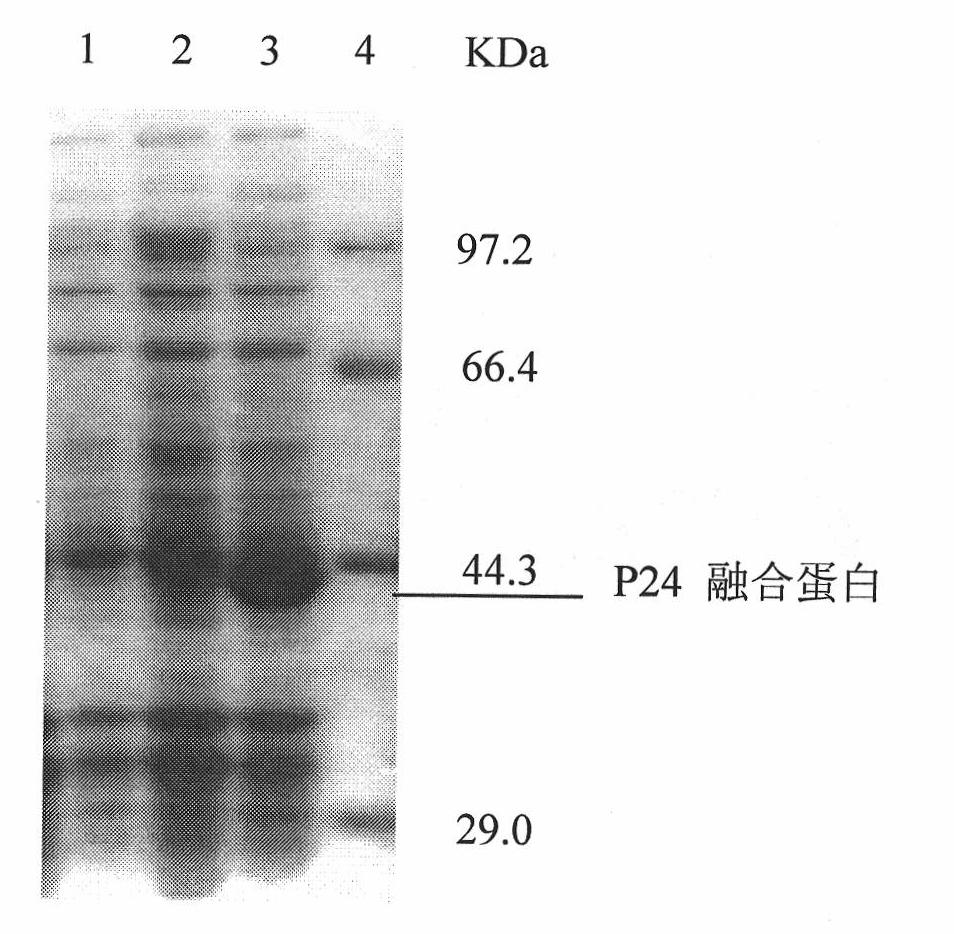
The invention discloses a monoclonal antibody hybridoma cell expressing an HIV P24 and an application, wherein, through separating HIV virus in an HIV positive infector, the P24 protein gene of the separated virus is enlarged; P24 protein can be obtained through external expression of carriers and purification; after using the purified P24 protein to immunize the mice, the spleen B cells of the immunized mice are separated out, and an hybridoma cell efficiently expressing a P24 monoclonal antibody (whiov-P24C-MoAb, CCTCC NO:C200818) can be obtained by adopting a hybridoma technology; and the P24 monoclonal antibody can be in specific binding with P24 protein of HIV virus for detecting the expression of P24 antigen. The P24 monoclonal antibody can be in specific binding with P24 protein ofHIV virus, interferences of other bad reactions can be reduced when being used for specific detection of P24 protein.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Heteroduplex tracking assay
InactiveUS7344830B2Accurately predict disease prognosis over timeGood treatment effectMicrobiological testing/measurementVertebrate cellsHeterologousHeteroduplex
Owner:HEALTH RES INC
Drug for AIDS treatment
InactiveUS20020164343A1Snake antigen ingredientsMammal material medical ingredientsWhite blood cellLow T cell count
Raising the T-cell count in an HIV positive patient having a low T-cell count by orally administering an effective amount of a composition containing a material obtained by treating whole human blood or white cells obtained from HIV positive patients with cold aqueous carbon dioxide, heating to evolve carbon dioxide gas, allowing a precipitate to form, and collecting and drying the precipitate.
Owner:IMMUNITOR USA
Analysis of HIV-1 coreceptor use in the clinical care of HIV-1-infected patients
InactiveUS20040121318A1Improve efficacyReduce the possibilityMicrobiological testing/measurementVertebrate cellsImmunodeficiency virusHIV positives
A change in viral tropism occurs in many HIV positive individuals over time and can be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus can be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CXCR4 specific strains. The diagnostic methods can be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time.
Owner:HEALTH RES INC
Uses of nordihydroguaiaretic acid (NDGA) and derivatives thereof in preventing transmission of sexually transmitted diseases
InactiveUS20090156638A1Prevent proliferationInhibitory activityBiocideHydroxy compound active ingredientsDihydroguaiaretic acidHIV positives
Provided herein are methods for inhibiting proliferation of a microbe associated with a sexually transmitted disease (STD) or infection and for preventing transmission of one or more sexually transmitted diseases in a subject at-risk for acquiring the same, including an HIV positive subject at risk for acquiring another sexually transmitted disease. The methods comprise contacting a microbe or cell thereof associated with the STD or administering to the subject nordihydroguaiaretic acid or a derivative or analog thereof. In HIV positive at-risk subjects one or more anti-retroviral drugs are co-administered.
Owner:KHANNA NIHARIKA
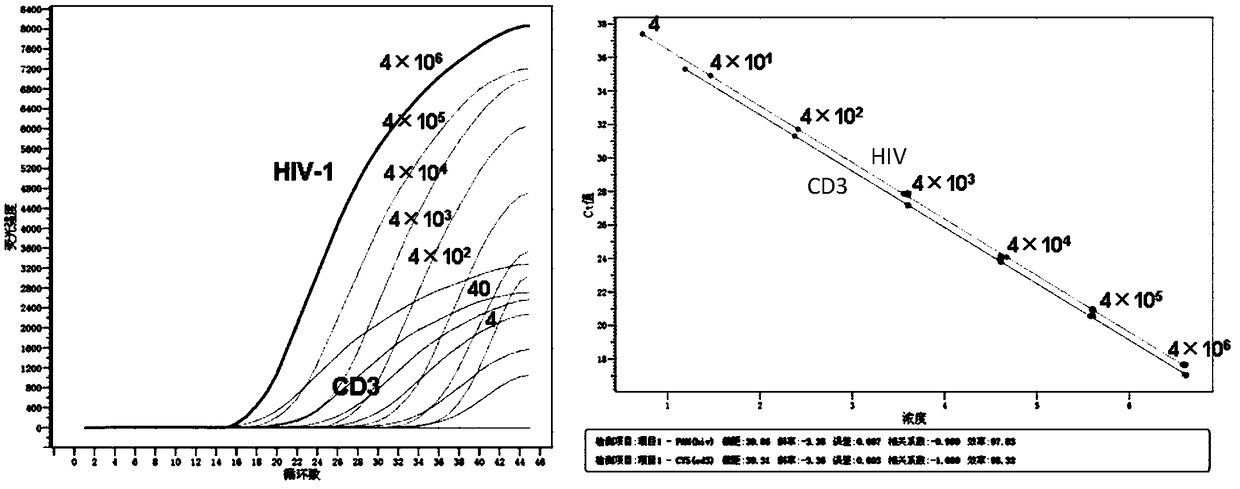
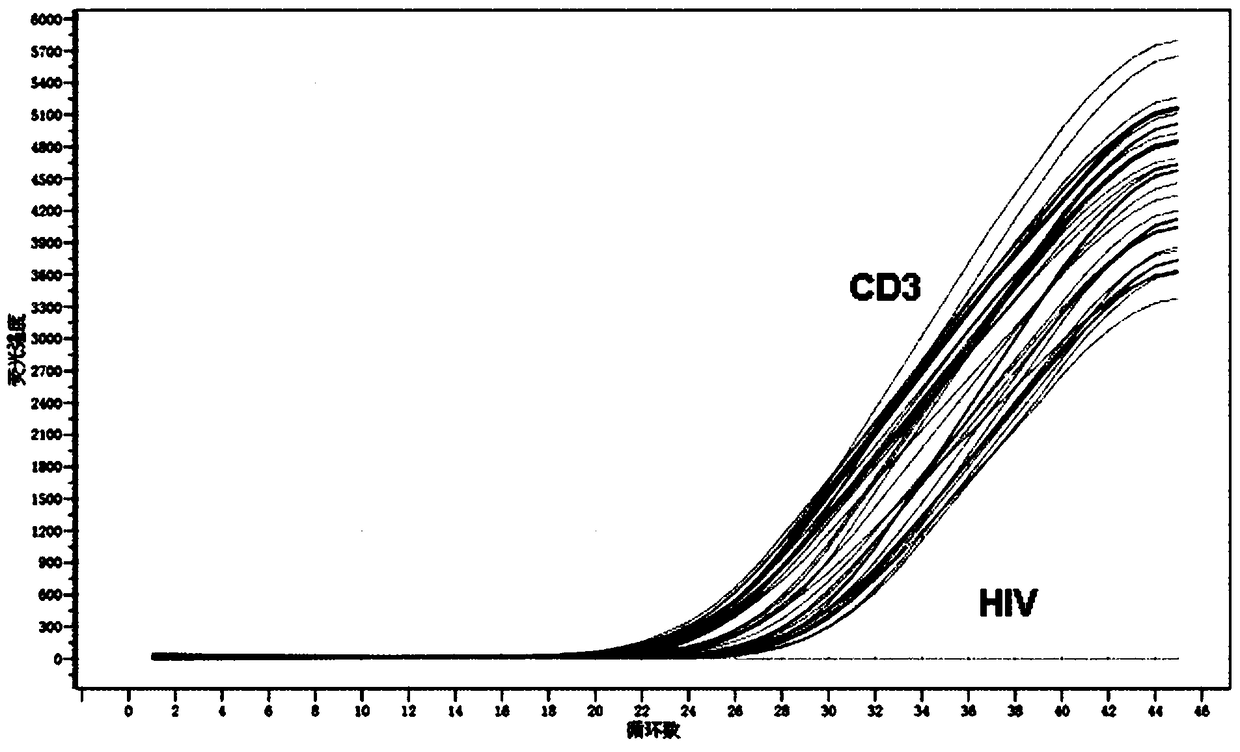
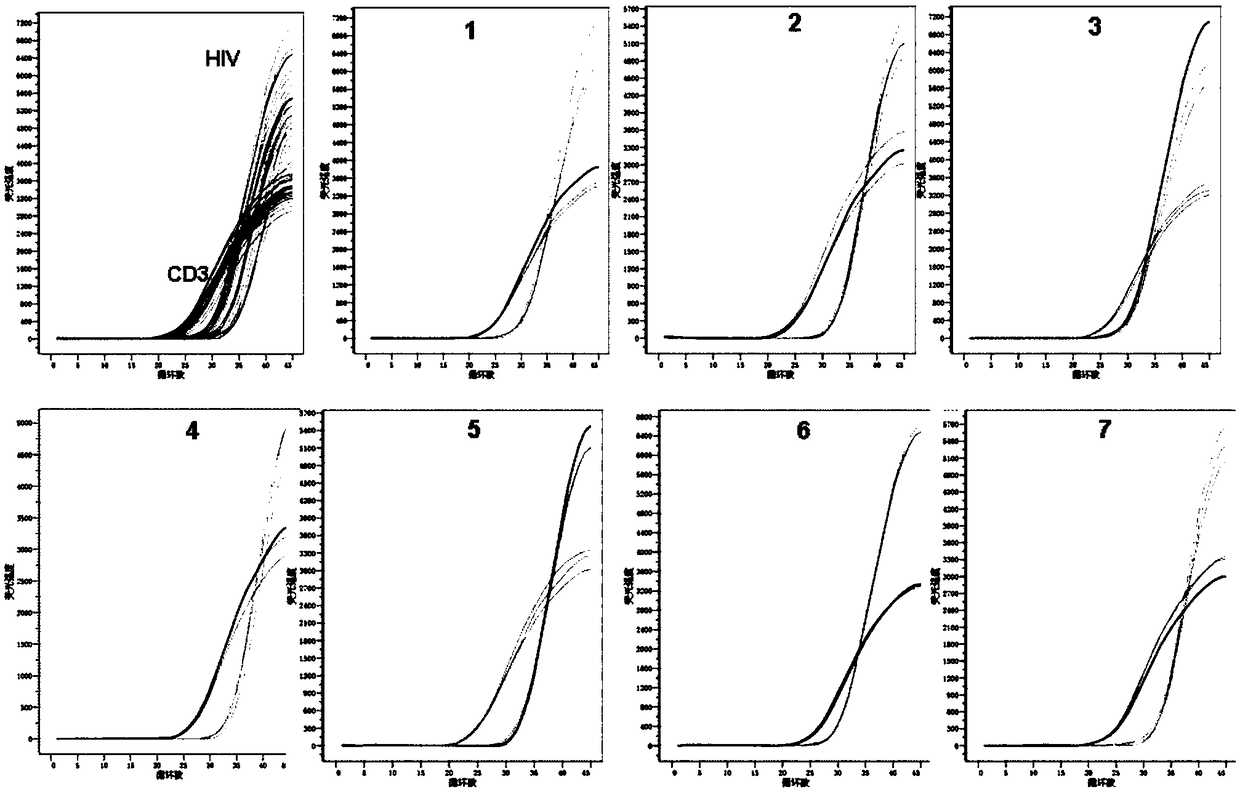
Primer pair, probe and kit used for quantitative detection total DNAs of HIV-1 and capable of covering multiple subtypes of HIV-1
ActiveCN108998570ABroad Subtype Detection CoverageOvercome operabilityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceHIV positives
The invention discloses a primer pair, probe and kit used for quantitative detection total DNAs of HIV-1 and capable of covering multiple subtypes of HIV-1. The sequence of the upstream primer HIV-U1of the primer pair is as shown in SEQ ID No. 1; the sequence of the downstream primer HIV-R1 of the primer pair is as shown in SEQ ID No. 2; the sequence of the probe Probe-HIV is as shown in SEQ ID No. 3. According to the invention, degenerate primers are utilized for high-sensitivity high-specificity detection of HIV DNA, and 12 common subtypes of HIV and rare HIV subtypes can be successfully detected, so the detection kit of the invention has wider subtype detection coverage compared with other conventional commercial detection kits. The primer pair, probe and kit provided by the inventioncan be used for quantification of total HIV DNAs of current prevalent HIV strains in China. A method provided by the invention is simple to operate, can complete procedures from sample DNA extractionto real-time fluorescent quantitative PCR amplification in one day, has low price, can be used for monitoring the persistent infection status of HIV-infected individuals in large cohorts, evaluating the effect of cART treatment, and is also applicable to auxiliary diagnosis of early HIV infection and diagnosis of neonatal infection in HIV-positive mothers.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV
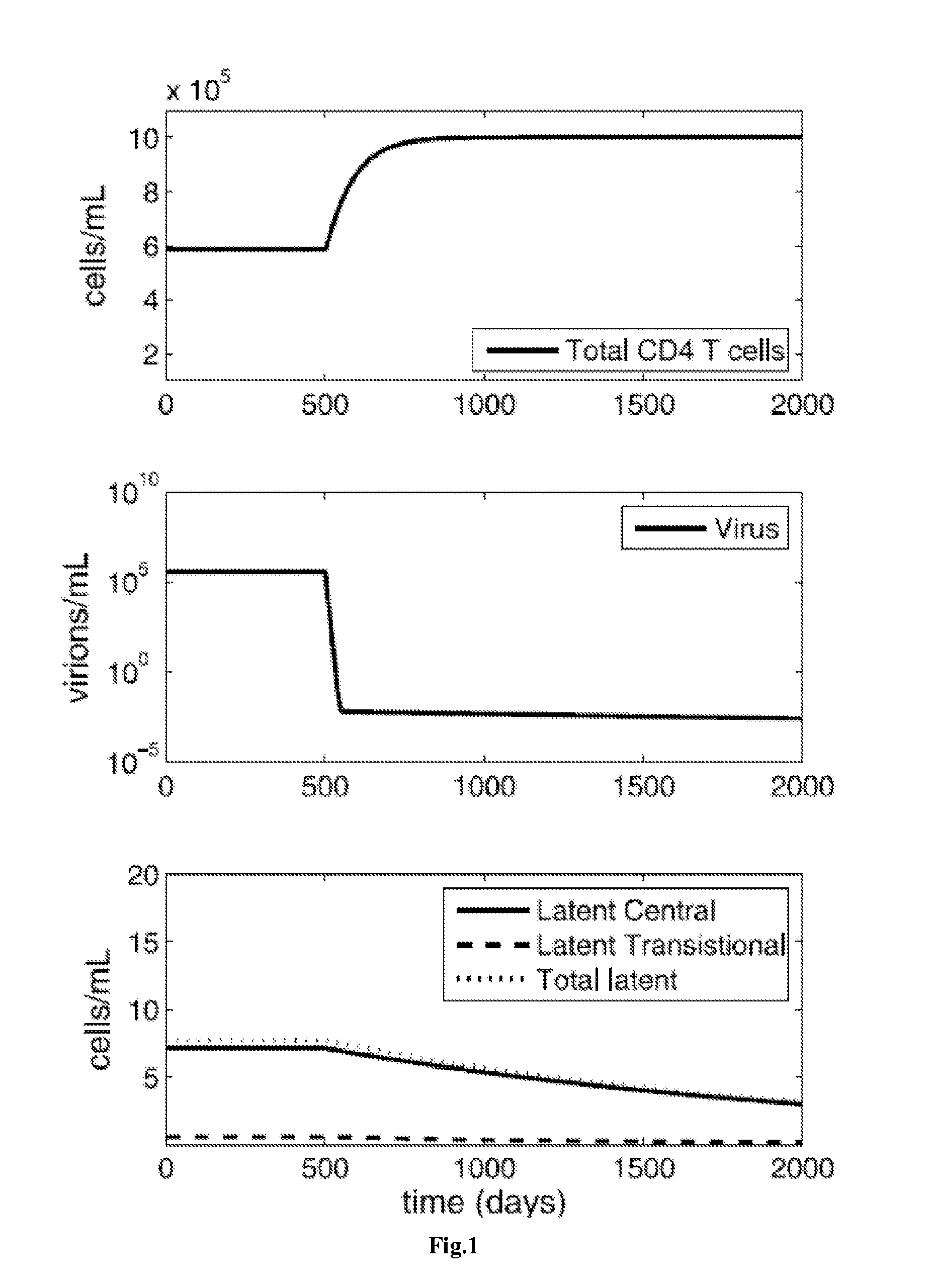
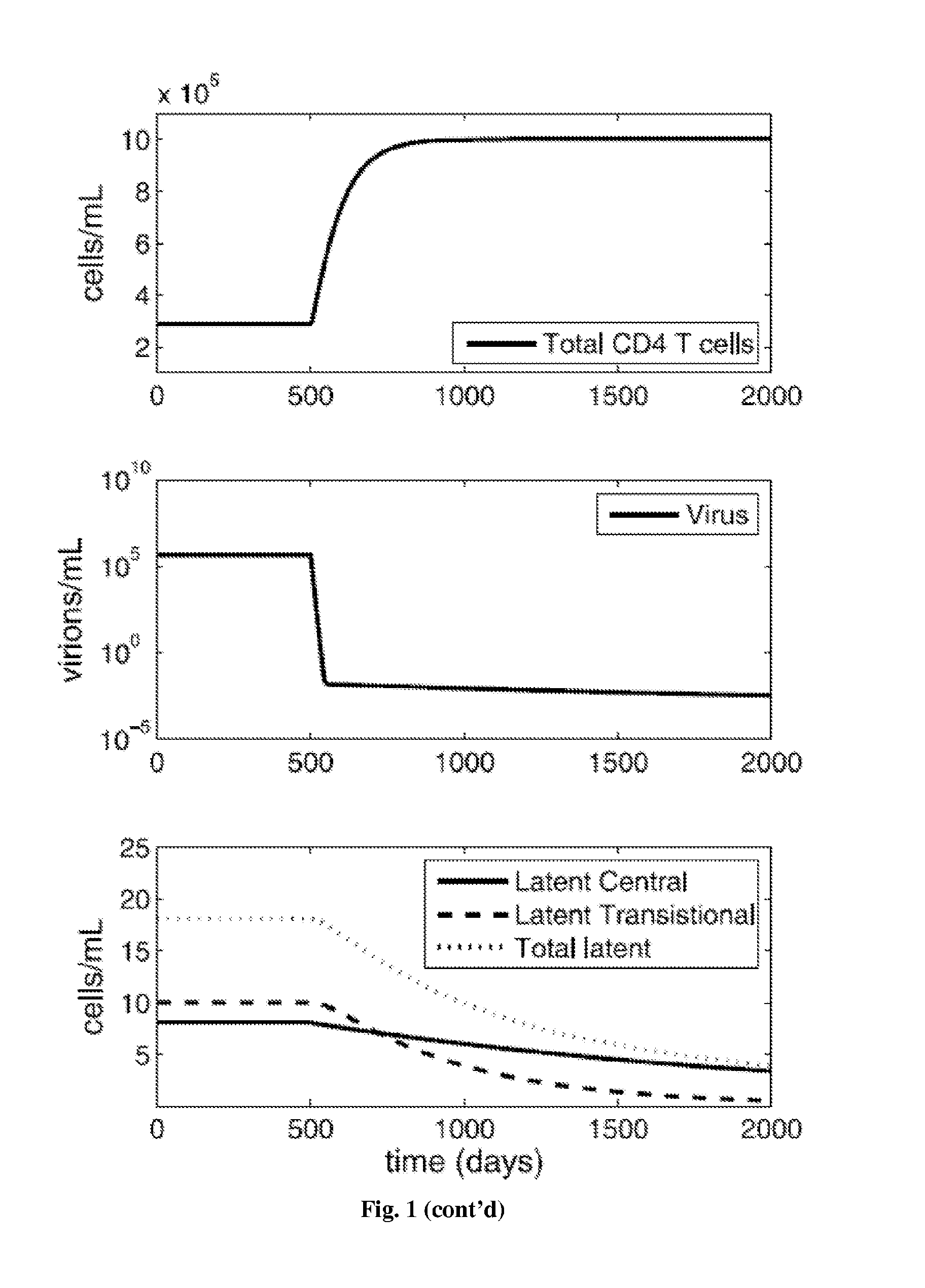
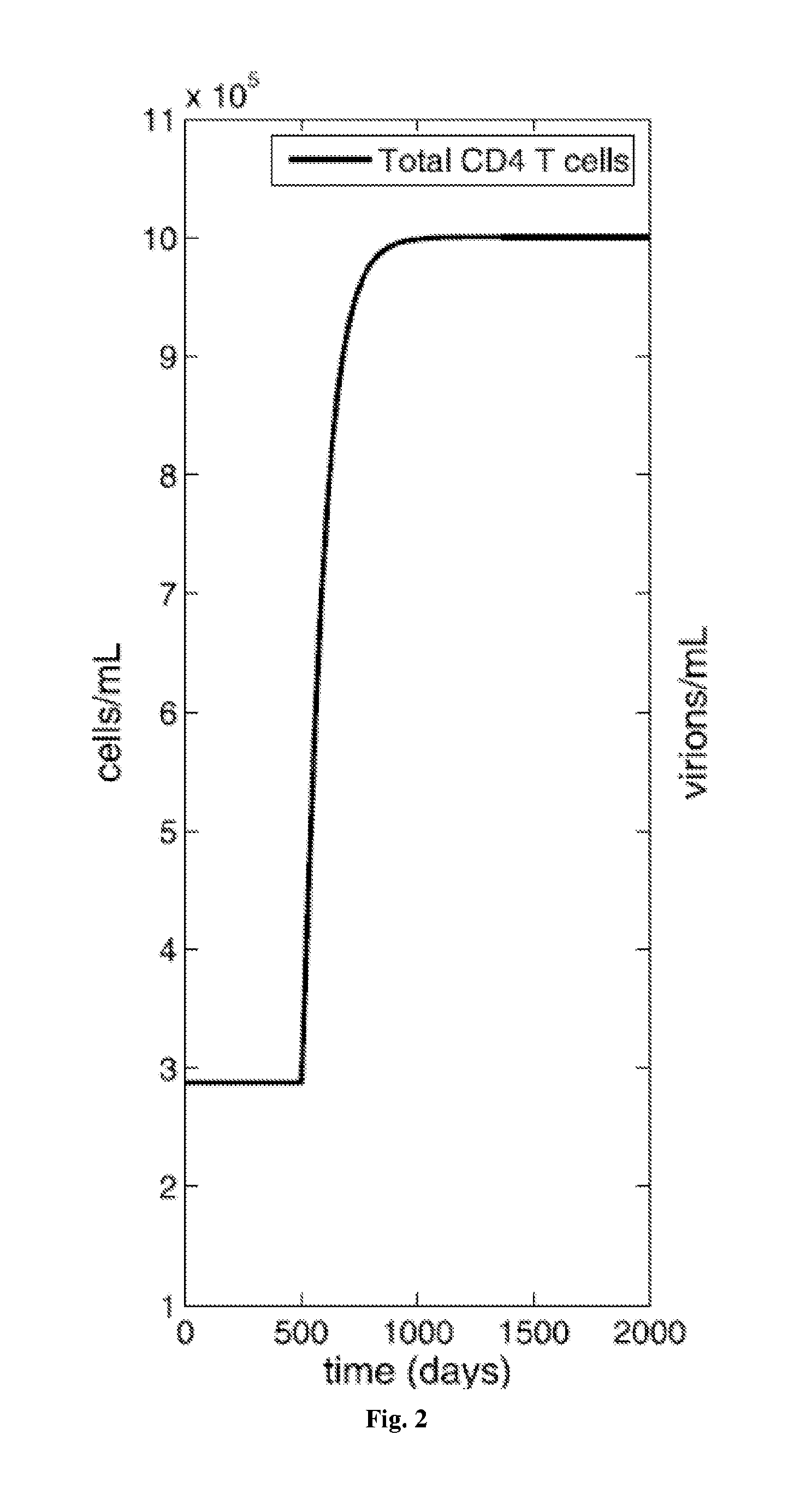
Treatment of latent HIV infection
InactiveUS20150320893A1EliminateEncourage viral productionBiocideAntiviralsReverse transcriptaseProstaglandin analog
Methods for treating HIV positive patients, and purging and eradicating latent HIV virus from a patient's system, are disclosed. The bulk of viral load is eradicated using conventional antiretroviral (ARV) therapy. Compounds that encourage viral production in the latent cells are then administered, preferably without activating those cells, while maintaining the ARV therapy. The administration of compounds that encourage viral production in latent cells is cycled, and after around 7-10 cycles, the methods can virtually eliminate latent HIV in the patient. Ideally, the ARV regimen includes at least one integrase inhibitor, at least one entry inhibitor, such as a CCR5 antagonist, and at least one, and preferably two, reverse transcriptase inhibitors. The compounds that encourage viral production in latent cells ideally include a combination of prostratin or a prostratin analog and an HDAC inhibitor, such as butyrate, valproate, or SAHA.
Owner:VOLPE JOSEPH M
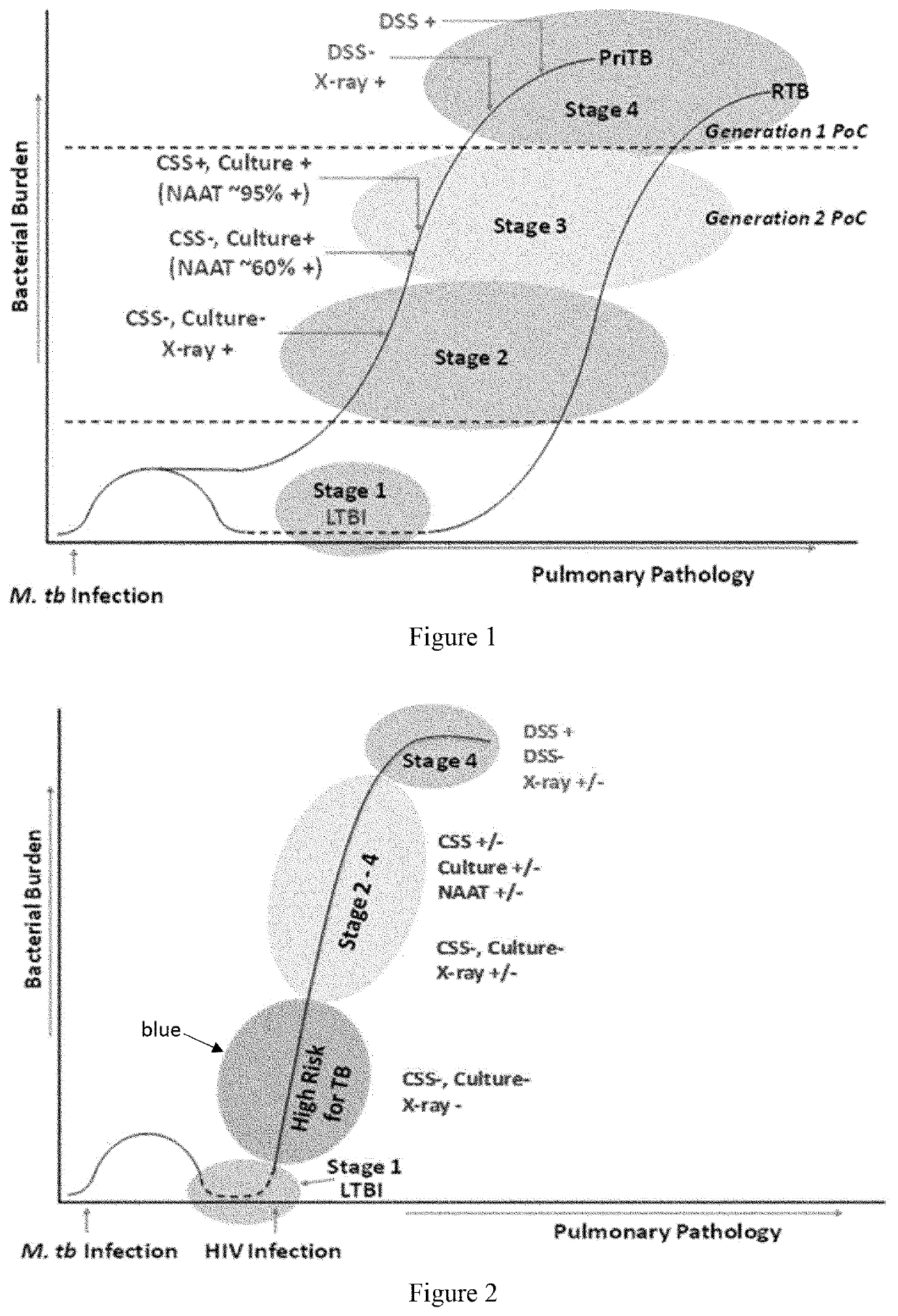
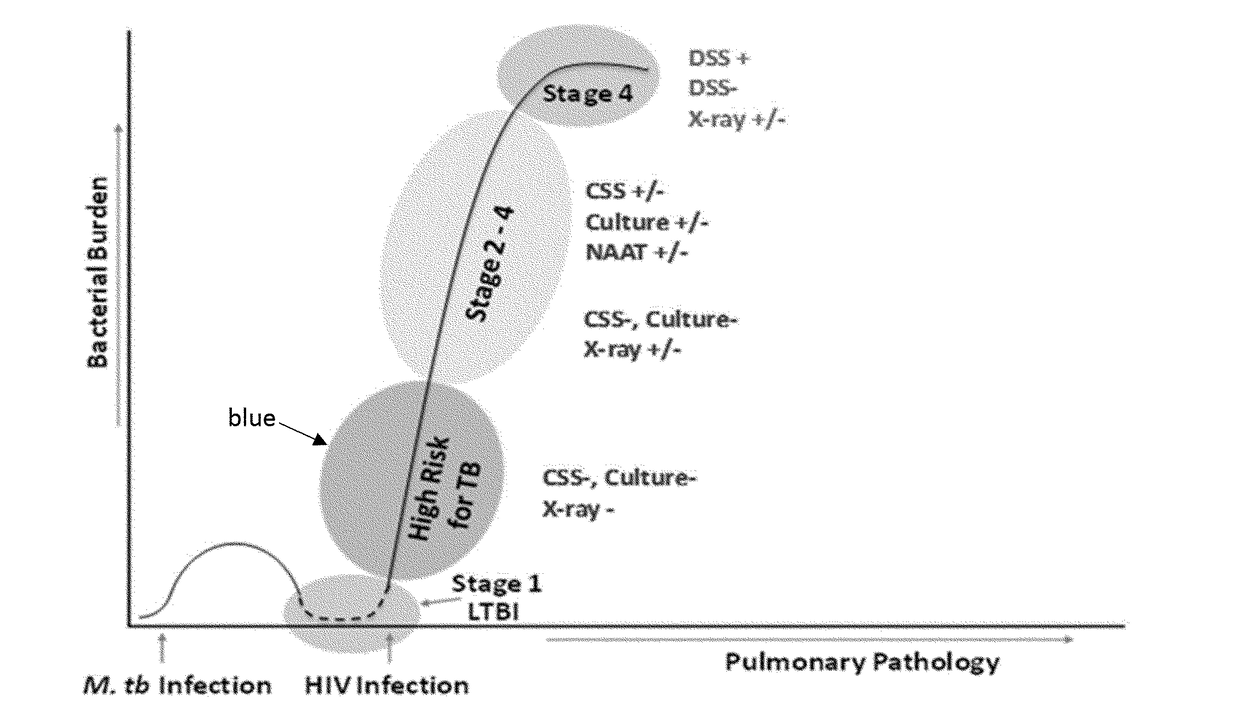
Peptides of m. tuberculosis for a screening test for HIV positive patients at high-risk for tuberculosis
Provided are peptides suitable for early detection of active M. tuberculosis (Mtb) infection in immunocompromised individuals. The peptides can form complexes with antibodies directed to Mtb antigens MS, MPT51, ESAT6 or CFPIO. Also provided are methods for detected of complexes of the peptides and the antibodies. The presence of complexes aids in predicting risk in immunocompromised individuals of developing active tuberculosis.
Owner:NEW YORK UNIV
Composition of anti-HIV drugs and anti-cortisol compounds and method for decreasing the side effects of anti-HIV drugs in a human
InactiveUS7354906B2Elevated triglycerideElevated cholesterolBiocideSugar derivativesProcaineSide effect
The present invention is based, in part, upon the discovery that the use of an anti-HIV drug in combination with at least one cortisol blocker such as procaine, reduces the side effects associated with anti-HIV drugs. The invention also relates to a method of treating the high cortisol catabolic effects of diseases such as AIDS in the HIV positive population and those with AIDS related complexes by the administration of a cortisol blocker. The present invention also discloses a composition comprising an anti-HIV drug and cortisol blocker. More specifically, the present invention relates to a cortisol blocking composition which comprises procaine, ascorbic acid and zinc heptahydrate.
Owner:SAMARITAN PHARMA
Crystal forms of 1-[5-methanesulfonamidoindolyl-2-carbonyl]-4-[3-(1-methylethylamino)-2-pyridinyl]piperazine
InactiveUS6452007B1Accurately determineOrganic active ingredientsOrganic chemistryHIV positivesPiperazine
The present invention relates to two novel crystal forms of a known compound, 1-[5-Methanesulfonamidoindolyl-2-carbonyl]-4-[3-(1-methylethylamino)-2-pyridinyl] piperazine monomethanesulfonate salt, which are useful for treating humans who are HIV positive, which are identified by a powder X-ray diffraction spectrum known commonly as the "S" and "T" forms.
Owner:PHARMACIA & UPJOHN CO +1
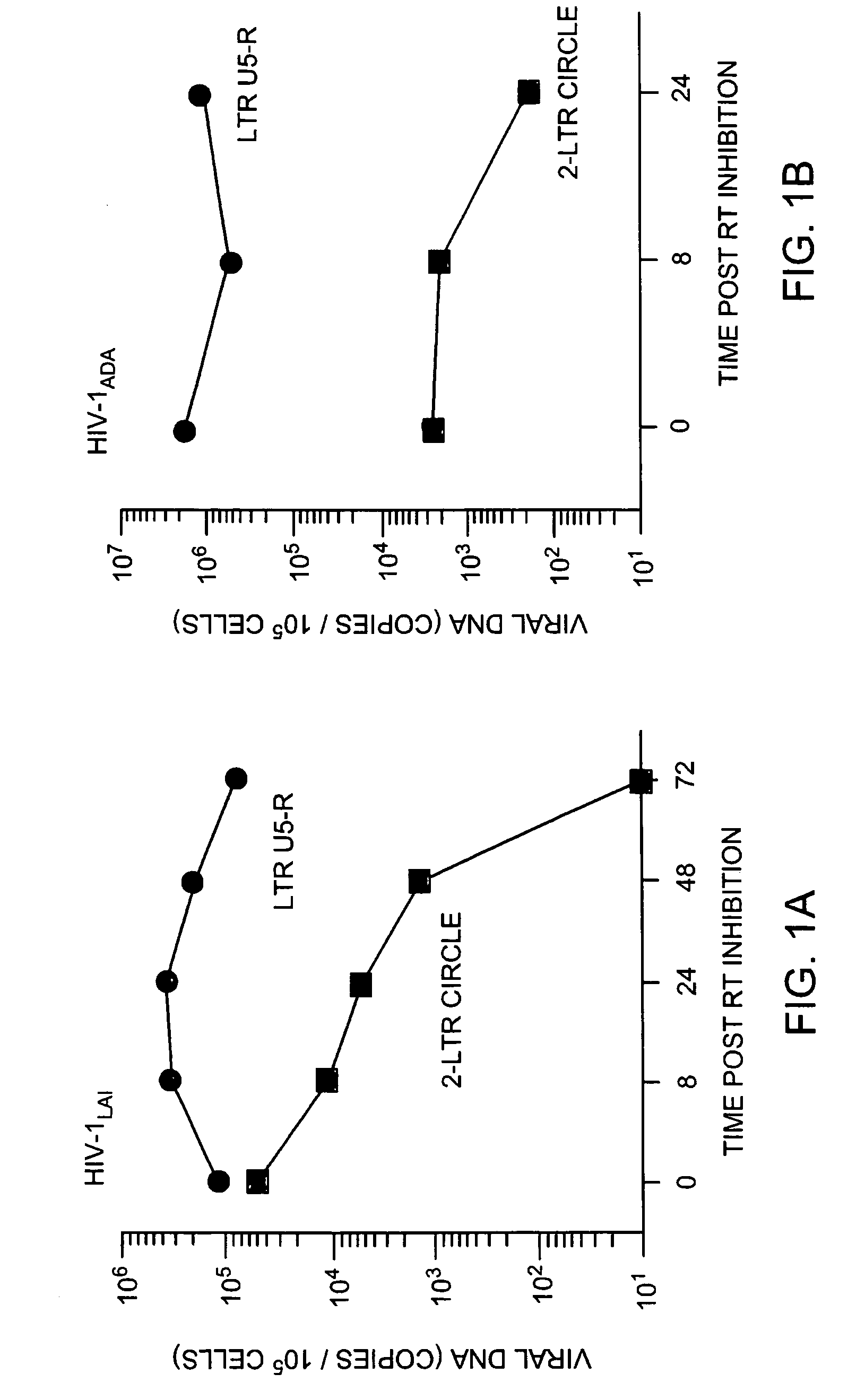
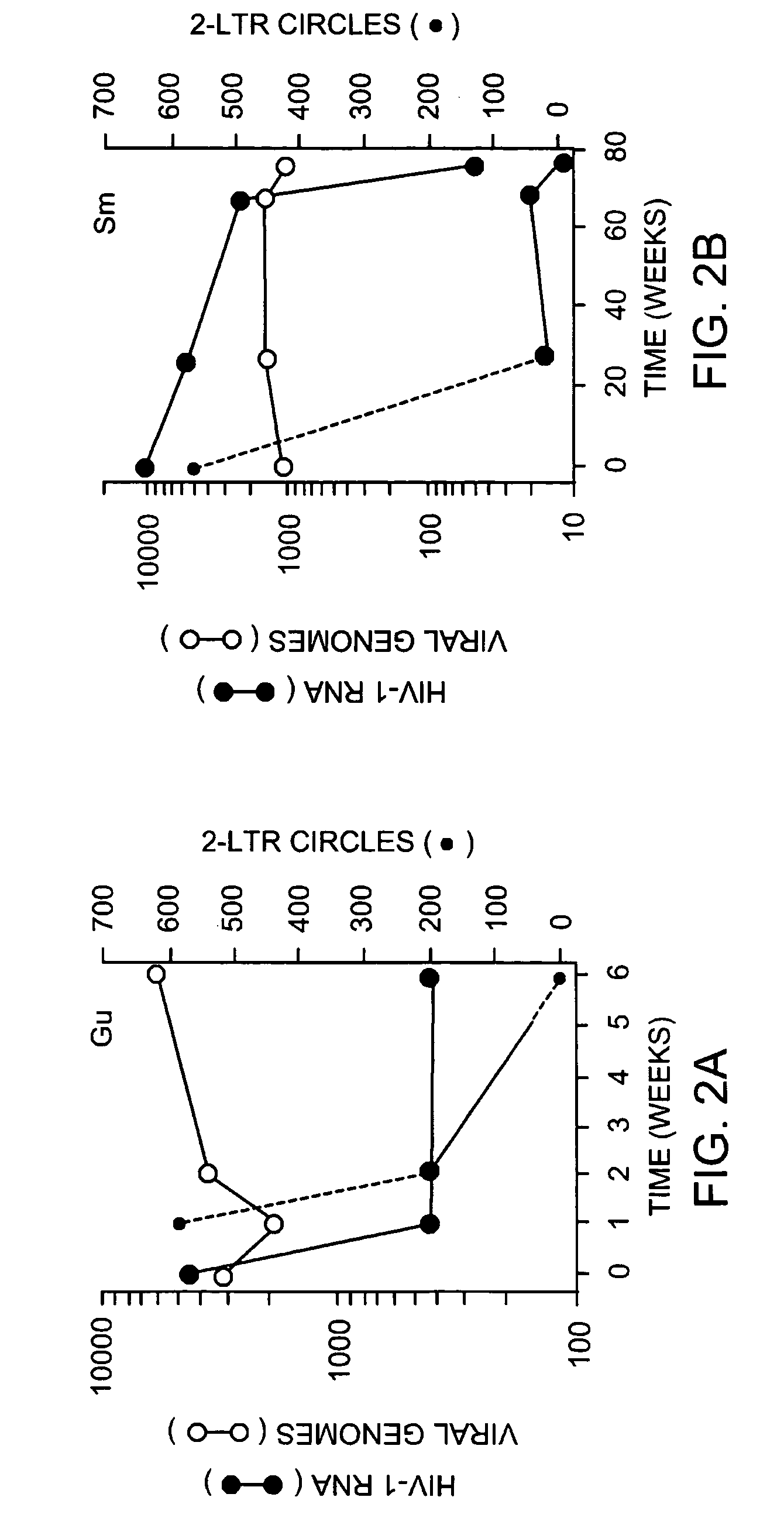
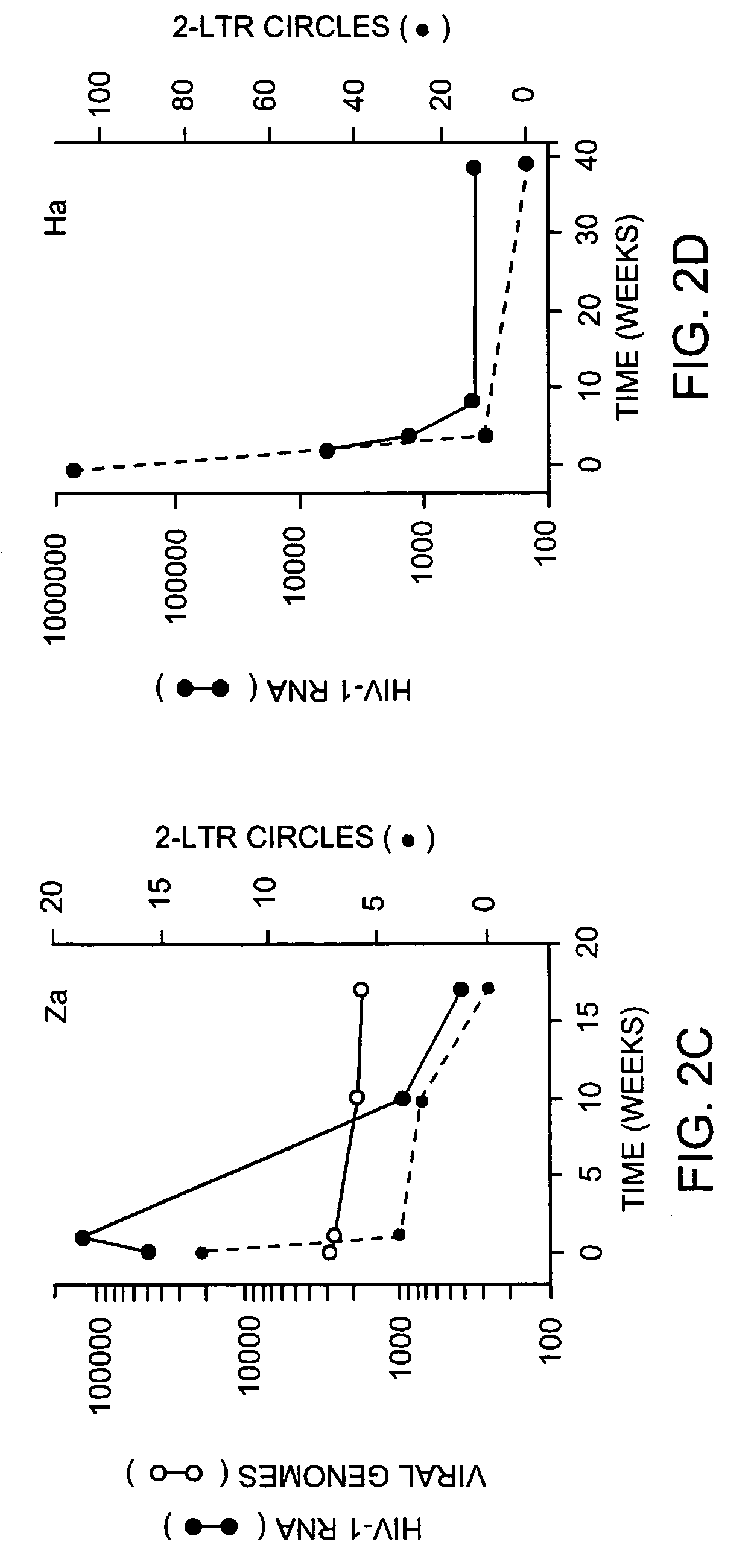
Detection of human immunodeficiency virus
InactiveUS7507527B2Devastating symptomPersistent infectionSugar derivativesMicrobiological testing/measurementMammalImmunodeficiency virus
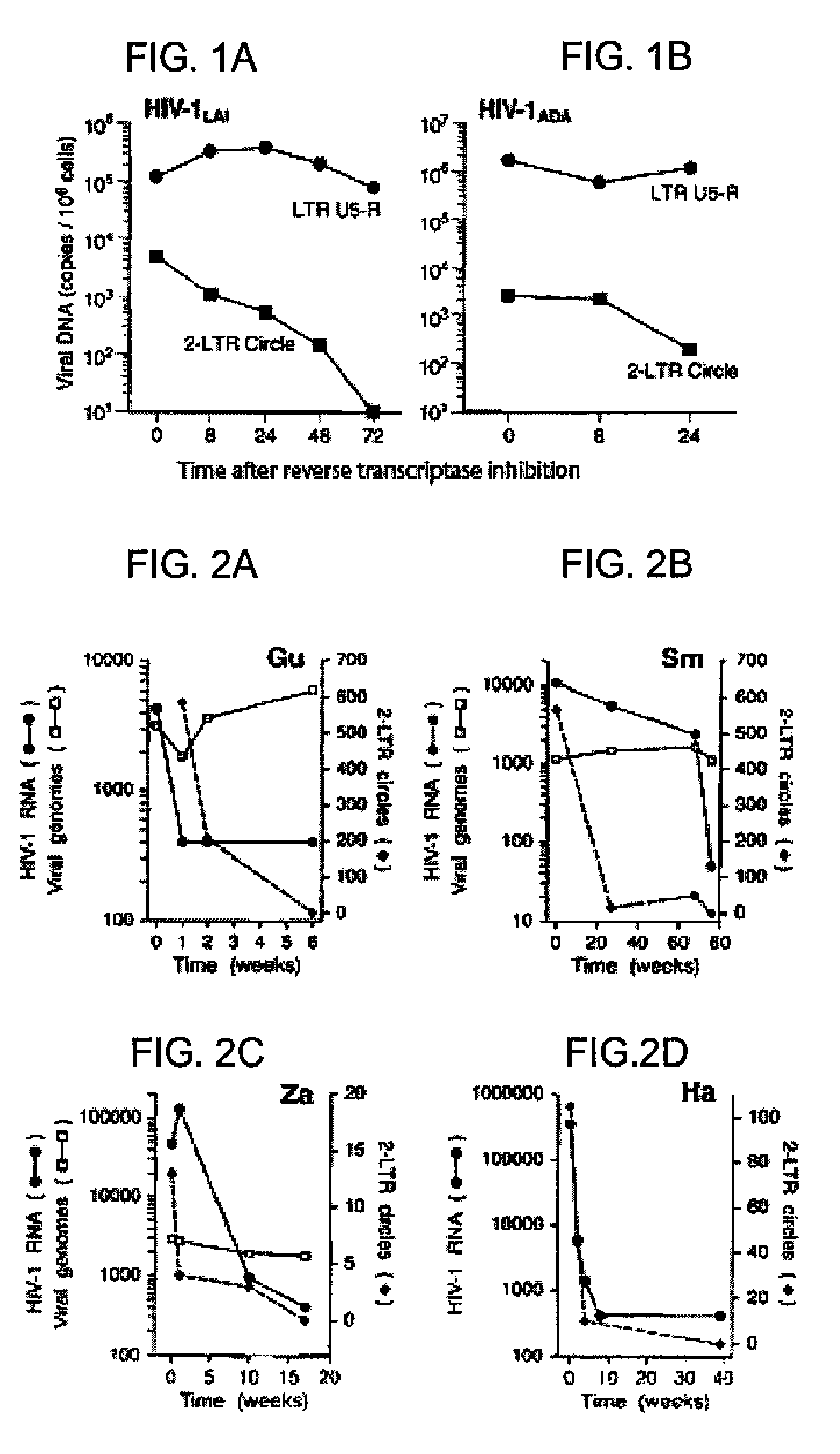
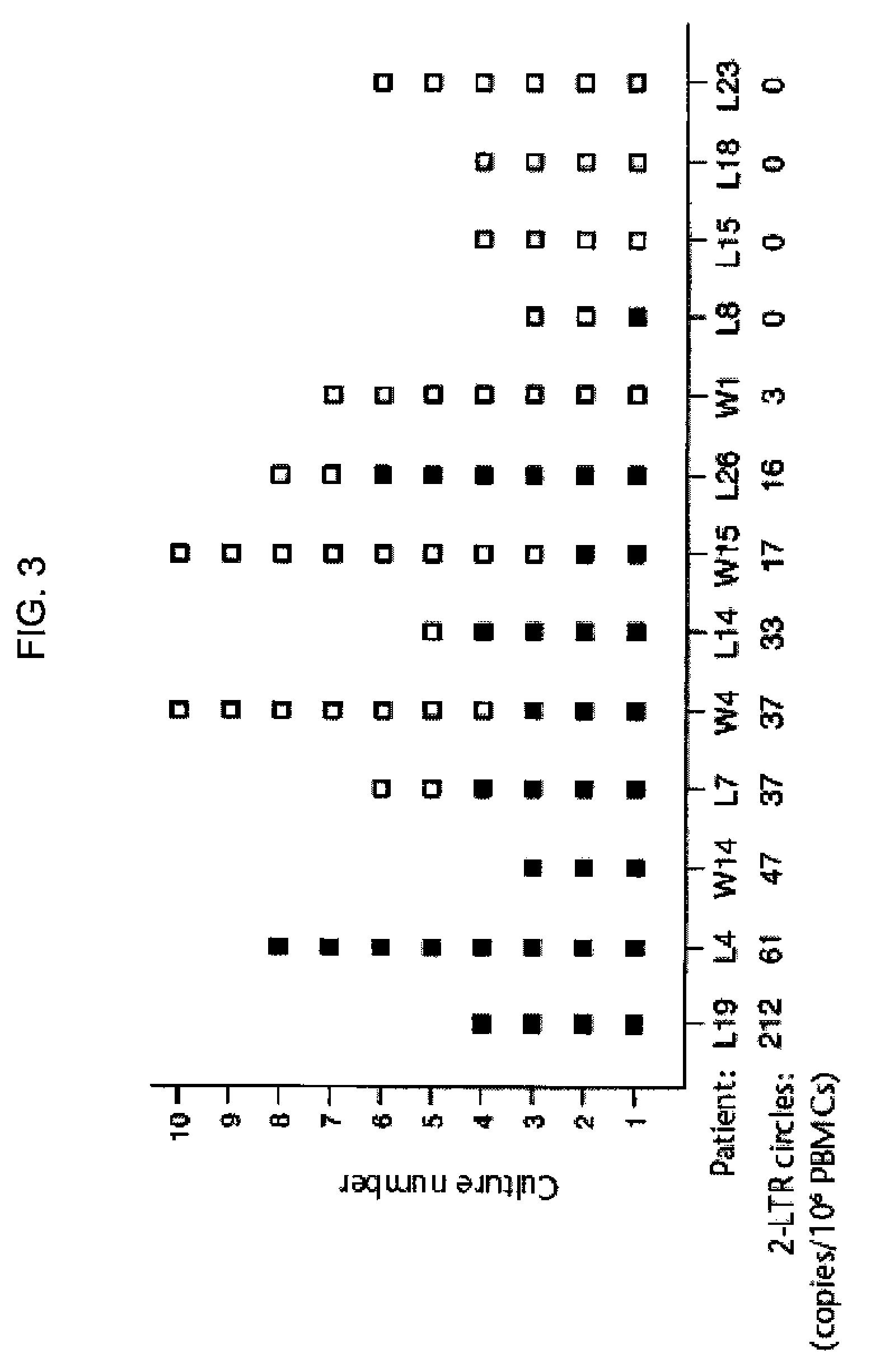
The invention relates to a method of detecting a HIV-infected cell in a mammal. The method includes detecting an HIV 2-LTR circle DNA molecule obtained from a cell of an HIV-positive mammal, especially an HIV-1-positive human.
Owner:THE PRESIDENTS OFFICE UNIV OF MASSACHUSETTS
Heteroduplex tracking assay
A change in viral tropism occurs in many HIV positive individuals over time and may be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus may be shifted back to CCR5-mediated entry soon after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment and clinical management of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CCR5- or CXCR4-specific strains. The diagnostic methods may be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time. The methods of the invention include cell-based methods, including cell fusion assays, and molecular-based methods, including heteroduplex tracking assay, to both quantitatively and qualitatively analyze patient-derived HIV for coreceptor usage.
Owner:HEALTH RES INC
Monoclonal antibody hybridoma cell of HIV P24 and application
InactiveCN101671655BApplicable testingIncreased sensitivityTissue cultureMaterial analysisSpecific detectionHIV positives
The invention discloses a monoclonal antibody hybridoma cell expressing an HIV P24 and an application, wherein, through separating HIV virus in an HIV positive infector, the P24 protein gene of the separated virus is enlarged; P24 protein can be obtained through external expression of carriers and purification; after using the purified P24 protein to immunize the mice, the spleen B cells of the immunized mice are separated out, and an hybridoma cell efficiently expressing a P24 monoclonal antibody (whiov-P24C-MoAb, CCTCC NO:C200818) can be obtained by adopting a hybridoma technology; and the P24 monoclonal antibody can be in specific binding with P24 protein of HIV virus for detecting the expression of P24 antigen. The P24 monoclonal antibody can be in specific binding with P24 protein ofHIV virus, interferences of other bad reactions can be reduced when being used for specific detection of P24 protein.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
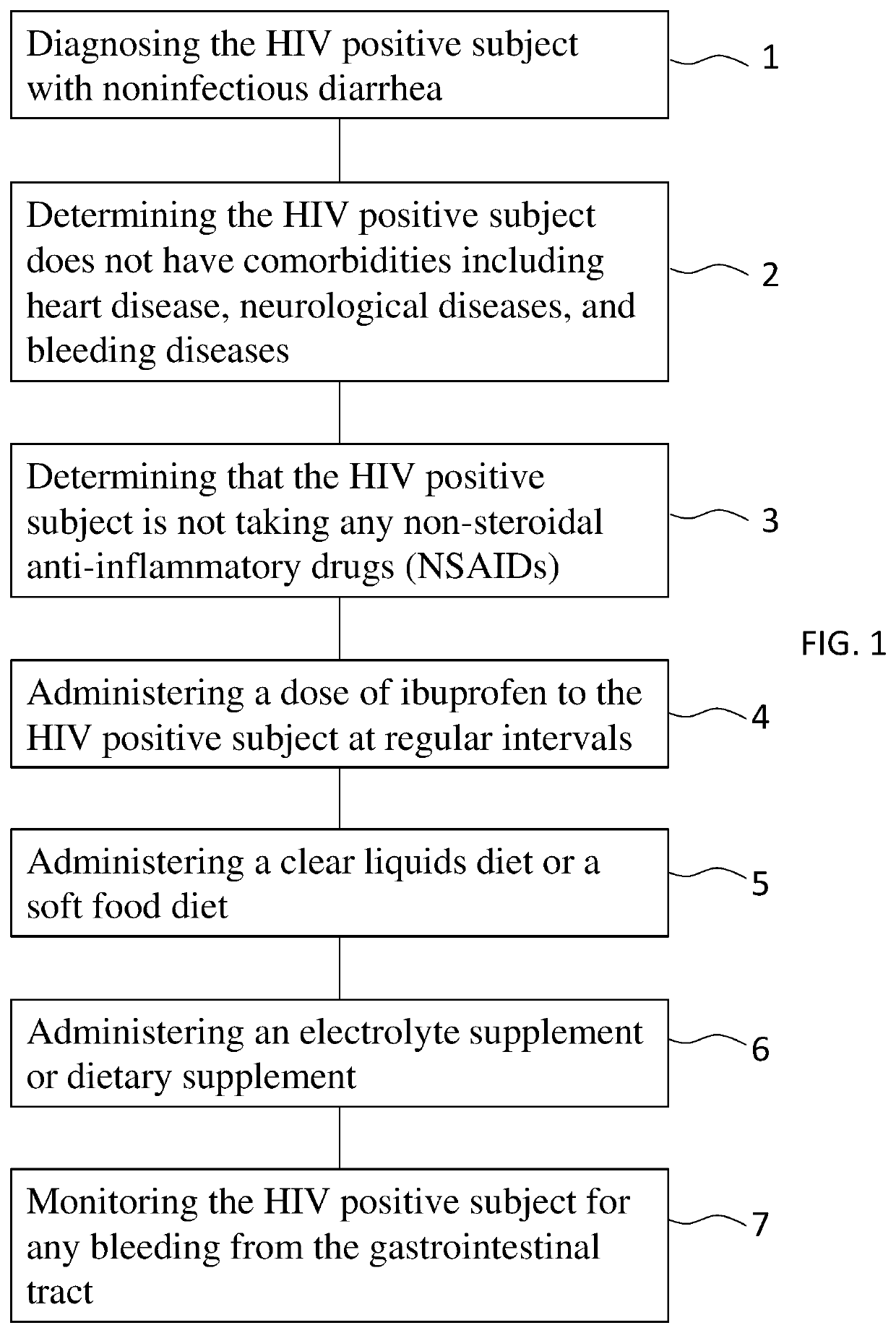
Ibuprofen for symptomatic treatment of diarrheas in HIV patients
PendingUS20220304961A1Stool consistencyReduce in quantityOrganic active ingredientsDigestive systemNon steroid anti inflammatory drugHIV positives
A method of treating noninfectious diarrhea in an HIV positive subject includes administering a dose of ibuprofen to the HIV positive subject at regular intervals until stool consistency is improved, diarrhea is alleviated, or the number of bowel movements per day are decreased. The dose is 400 mg of ibuprofen administered every 6 hours. The method may additionally include diagnosing the subject with noninfectious diarrhea, determining the subject does not have certain comorbidities, and / or determining that the subject is not taking any non-steroidal anti-inflammatory drugs. The method may additionally include after administering the ibuprofen, administering a clear liquids diet to the subject, administering an electrolyte supplement to the subject, and / or monitoring the subject for any bleeding from the gastrointestinal tract until stool consistency is improved, until diarrhea is alleviated, or until the number of bowel movements per day are decreased.
Owner:NIBHANIPUDI KUMARA V
Peptides of M. tuberculosis for a screening test for HIV positive patients at high-risk for tuberculosis
Provided are peptides suitable for early detection of active M. tuberculosis (Mtb) infection in immunocompromised individuals. The peptides can form complexes with antibodies directed to Mtb antigens MS, MPT51, ESAT6 or CFPIO. Also provided are methods for detected of complexes of the peptides and the antibodies. The presence of complexes aids in predicting risk in immunocompromised individuals of developing active tuberculosis.
Owner:NEW YORK UNIV
Drug for AIDS treatment
InactiveUS7384637B2Raise CD- and CD- levelSnake antigen ingredientsMammal material medical ingredientsWhite blood cellLow T cell count
Owner:IMMUNITOR USA
Methods and compositions for optimizing blood and tissue stability of camptothecin and other albumin-binding therapeutic compounds
InactiveUS7691872B2Enhance drug levelImprove biological effectBiocideAntiviralsNitrocamptothecinHIV positives
The present invention provides methods and formulations for optimizing the anti-cancer and anti-HIV activities of a camptothecin drug, including camptothecin and its related analogs including 9-aminocamptothecin and 9-nitrocamptothecin. The invention involves methodologies and formulations that limit human serum albumin-mediated reduction of the anti-cancer and anti-HIV effects of the camptothecins, and the methods and formulations provide combination therapies in which binding of the camptothecin agent to human serum albumin can be modulated by the administration of a competing agent that also binds human serum albumin. Reduced camptothecin drug binding to human serum albumin can result in elevated camptothecin free drug levels and thus improve the effectiveness of treatment regimens involving these drugs. Further agents such as methotrexate and AZT can also be used in cancer and HIV-positive patients employing camptothecin drugs.
Owner:UNIV OF KENTUCKY RES FOUND +1
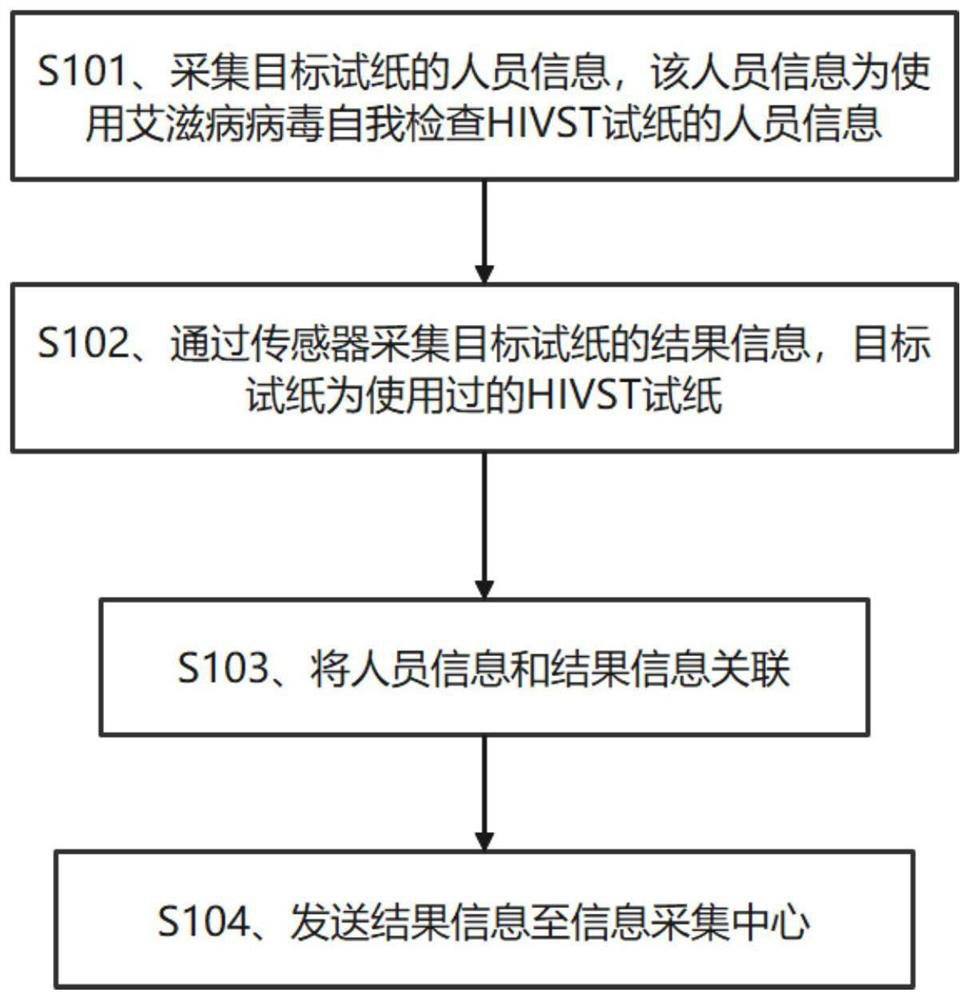
Information acquisition method, information acquisition equipment and storage medium
PendingCN113643775AMaterial analysis by observing effect on chemical indicatorPatient-specific dataHIV positivesEngineering
The invention provides an information collection method and information collection device. The information collection method comprises the following steps: collecting detection result information of target test paper through a sensor, wherein the target test paper is used human immunodeficiency virus self-detection HIVST test paper; and sending the detection result information to an information acquisition center. The information acquisition center can acquire the detection result information of the target test paper, and solved are the problems that as most self-checking motives have high-risk behaviors, and potential infected people cannot be primarily screened and rechecked in time due to condition limitation, the estimation of the HIV positive epidemic condition possibly has large deviation, a target population of HIVST, i.e., a sexual minority population, especially a high-risk population with HIV infection risk between male and male sexual behavior persons, is low in consciousness when the population returns a self-inspection result to health service personnel, and cannot effectively obtain real data after the self-inspection population is subjected to HIVST.
Owner:GUANGXI MEDICAL UNIVERSITY
Rapid detection method, liquid chip and kit for four human infectious diseases
PendingCN111206116AEasy to detectAccurate detectionMicrobiological testing/measurementSyphilisReverse transcriptase
The invention discloses a rapid detection method, liquid chip and kit for four human infectious diseases, and relates to the technical field of life sciences and biology. The liquid chip mainly includes 60-100 parts of a sample diluent, 10-20 parts of oligonucleotides for HIV detection, 5-10 parts of HIV positive control, 0.1-0.2 part of insulin, 20-50 parts of amino acids, 0.2-0.5 part of DNA polymerase, 0.2-0.5 part of reverse transcriptase, 0.2-0.5 part of RNA polymerase, 10-20 parts of a nucleic acid releasing agent and 5-10 parts of nucleotide-targeted detection primers. According to therapid detection method, liquid chip and kit for the four human infectious diseases, high activity of cells and viruses can be maintained through coordination of the insulin and amino acids when AIDS,syphilis, hepatitis C and hepatitis B are detected, and the process of reactions of AIDS, syphilis, hepatitis C and hepatitis B viruses can be catalyzed and accelerated through combination of the DNApolymerase, reverse transcriptase and RNA polymerase, so that the detection effect of the detection kit is increased greatly, and the detection effect is more accurate.
Owner:武汉尚码生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Crystal forms of 1-[5-methanesulfonamidoindolyl-2-carbonyl]-4-[3-(1-methylethylamino)-2-pyridinyl]piperazine Crystal forms of 1-[5-methanesulfonamidoindolyl-2-carbonyl]-4-[3-(1-methylethylamino)-2-pyridinyl]piperazine](https://images-eureka.patsnap.com/patent_img/6d35421f-0138-4c2e-b967-abdf2053c4ca/US06452007-20020917-C00001.png)