Uses of nordihydroguaiaretic acid (NDGA) and derivatives thereof in preventing transmission of sexually transmitted diseases
a technology of nordihydroguaiaretic acid and derivatives, which is applied in the field of virology, bacteriology and public health, can solve problems such as deficiency in prophylactic and therapeutic methods in the early stages of the diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Microbicidal Compounds
[0049]Nordihydroguaiaretic Acid (NDGA)
[0050]NDGA is a purified extract of Larrea tridentata (Erimos Technologies).
[0051]NDGA Derivatives and Analogs
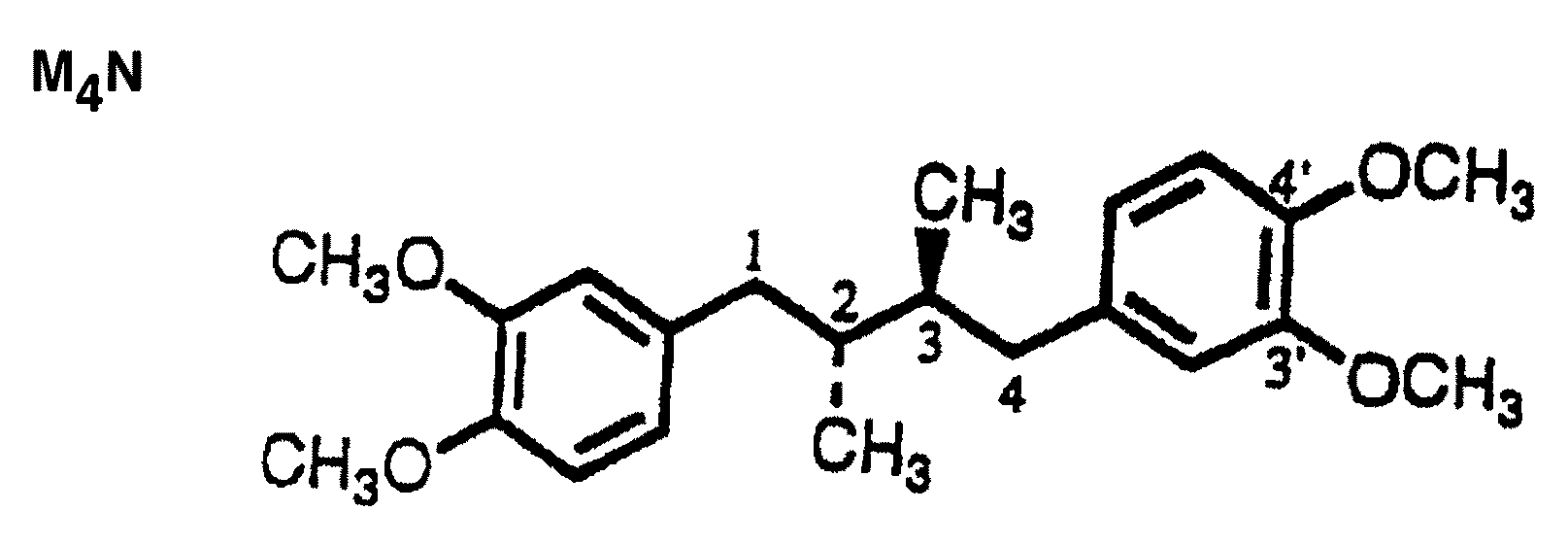
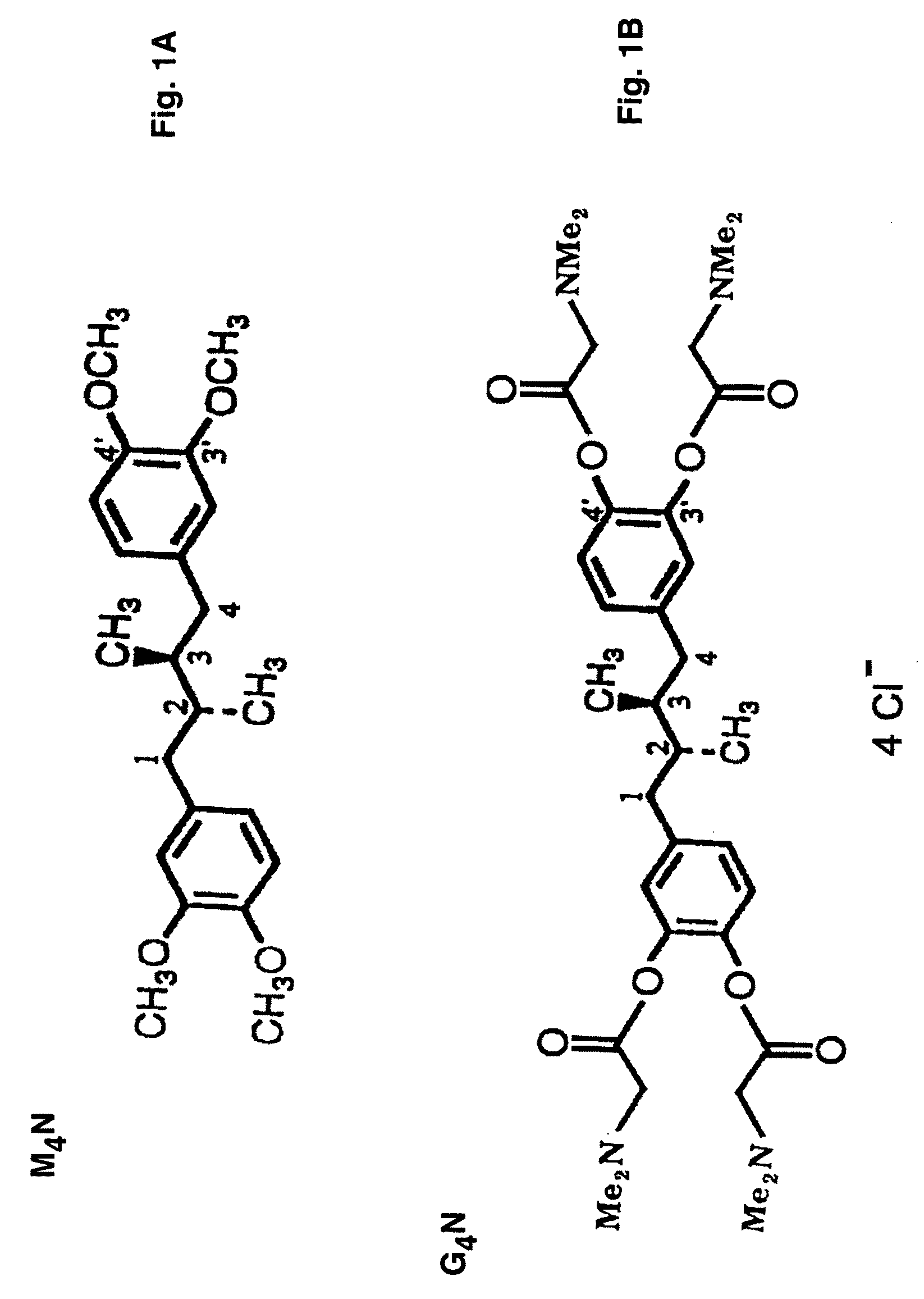
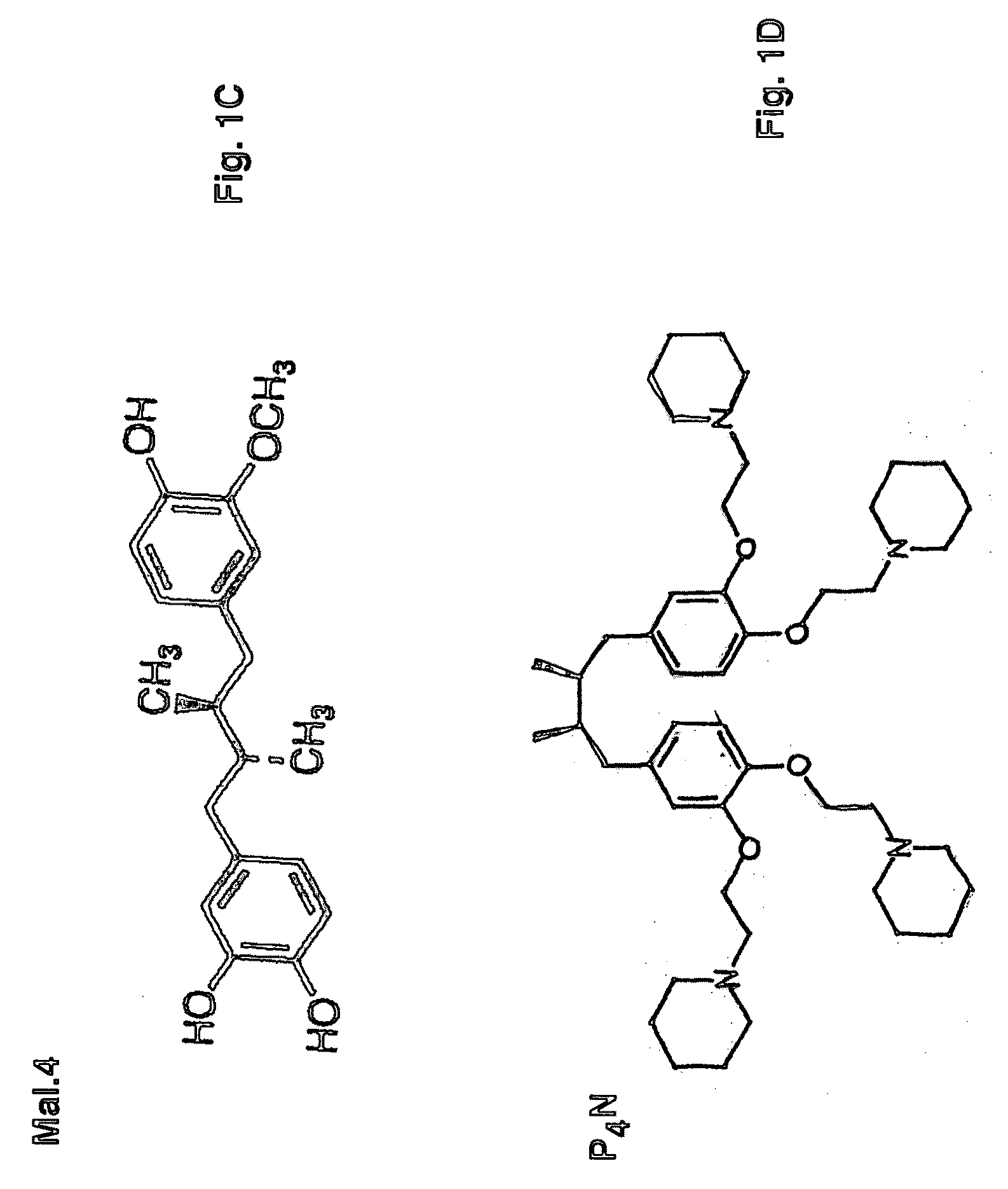
[0052]Tetra-O-methyl-nordihydroguaiaretic acid (M4N; FIG. 1A), tetra-O—(N,N-dimethyl)glycyl-nordihydroguaiaretic acid (G4N; FIG. 1B), 3′-O-methyl nordihydroguaiaretic acid (Mal.4; FIG. 1C), or tetra-O—(N-ethyl)piperidinyl-nordihydroguaiaretic acid (P4N) are derivatives of NDGA and are the active pharmaceutical ingredients of the microbicide. The microbicides may be hydrophobic, such as M4N, or water soluble such as G4N, Mal.4 or P4N.
[0053]Tetra-O-methyl-nordihydroguaiaretic acid (M4N) is a semi-synthetic derivative of NDGA synthesized by a two stage chemical methodology (Sigma Aldrich Fine Chemicals). tetra-O—(N,N-dimethyl)glycyl-nordihydroguaiaretic acid (G4N) is synthesized as described (3). 3′-O-methyl-nordihydroguaiaretic acid (Mal.4) also can be isolated from Larrea tridentata as disclosed in U.S. Pat. No. 6,29...
example 2
[0054]The screening algorithm depicted in FIG. 2 is used to develop the microbicide. In summary, an HIV R5 cell attachment assay is conducted with a potential microbicidal compound and followed with an R5 cell to cell transmission assay. If the compound is found to be active, the next stage of secondary screening occurs, in which an HIV X4 attachment and fusion assay is performed, as well as an HIV X4 cell to cell transmission assay. In addition, cervical explant studies and Lactobacillus toxicity testing are performed. HIV R5 cell to cell transmission assays in seminal plasma and assessment of effects on semen and sperm are also performed.
Antiviral Assays in Fresh Human Peripheral Blood Mononuclear Cells (PBMCs)
[0055]PBMCs are isolated following Ficoll-Hypaque density centrifugation, and re-suspended at a cell density of 1×106 in medium supplemented with 2 μg / mL PHA. Following incubation for 24 hrs, the cells are collected by centrifugation, washed and suspended ...
example 3
In Vitro Anti-Viral Activity
Sp1 Transcription Factor Binding
[0061]Tetra-O-dimethylaminoglycyl-nordihydroguaiaretic acid (G4N) binds the major grove of the free DNA when it is uncomplexed with other proteins. The binding constant for the G4N / Sp1 enhancer sequence was calculated by the ethidium displacement technique at various phosphate buffer concentrations and pH values using a spectrofluorometer with excitation at 517 nm and emission at 596 nm. An apparent equilibrium binding constant (Kapp) of 3×106 M−1 was obtained by the equation Kapp=KEtBr[EtBr] / [G4N] which represents the concentration of the G4N causing a 50% reduction of the fluorescent intensity of DNA-ethidium solution. Tetra-O—(N-ethylpiperidinyl)-nordihydroguaiaretic acid (P4N) has been found to disrupt Sp1 transcription factor binding to the major groove of Sp1 site either by competition for binding or by altering the overall DNA conformation such that the major groove becomes incompatible for Sp1 binding G4N has been t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com