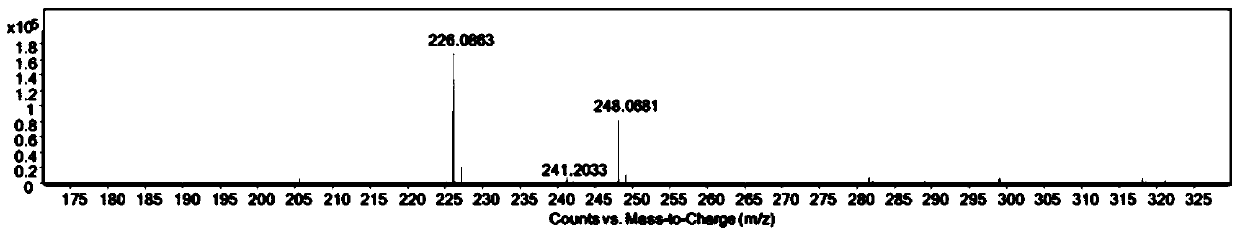
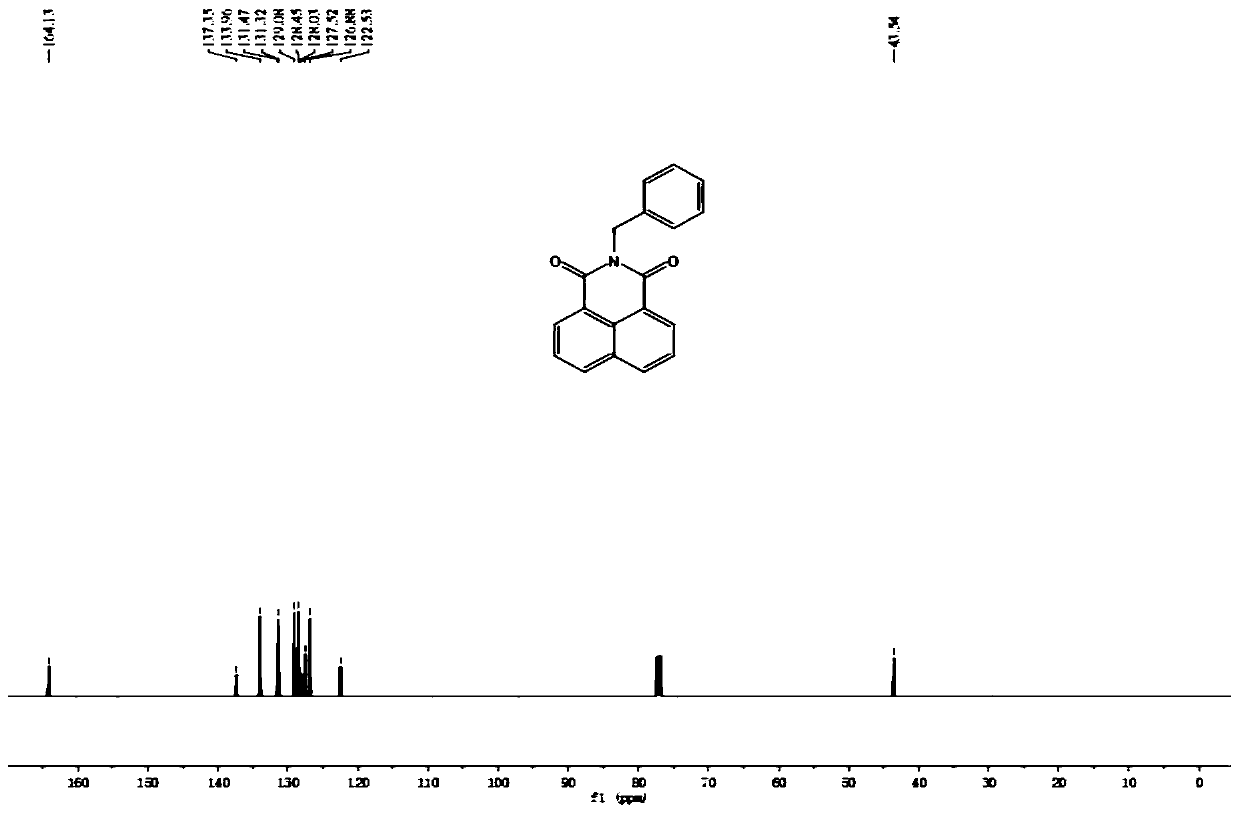
Patents
Literature
34 results about "Drug-drug interaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions & formulations for preventing and treating chronic diseases that cluster in patients such as cardiovascular disease, diabetes, obesity, polycystic ovary syndrome, hyperlipidemia and hypertension, as well as for preventing and treating other diseases and conditions
InactiveUS20140271923A1Good for healthImproving well-beingHeavy metal active ingredientsBiocideSide effectPolycystic ovary
Patients inflicted with various clustering chronic diseases require treatment with multiple drugs having distinct mechanisms of action. Accordingly, patients with multiple conditions suffer from cumulative side effects of multiple drugs as well as drug-drug interactions. Embodiments, agents, compounds or drugs of the present invention, such as sesquiterpenes, e.g., Zerumbone, replace an equal or larger number of approved drugs during patient treatment. Examples of disorders prevented or ameliorated by administration of the formulations of this invention include but are not limited to inflammatory diseases that may be, oncological, genetic, ischemic, infectious, neurological, hematological, ophthalmological, rheumatoid, orthopedic, neurological, hematological, kidney, vascular, dermatological, gynecological, or obstetric. The present invention further relates to a method of identifying agents, compounds or drugs useful in preventing or treating CDCP related diseases and conditions as well as other disorders, diseases and conditions treatable or preventable by the same agents, compounds or drugs.
Owner:REID CHRISTOPHER BRIAN
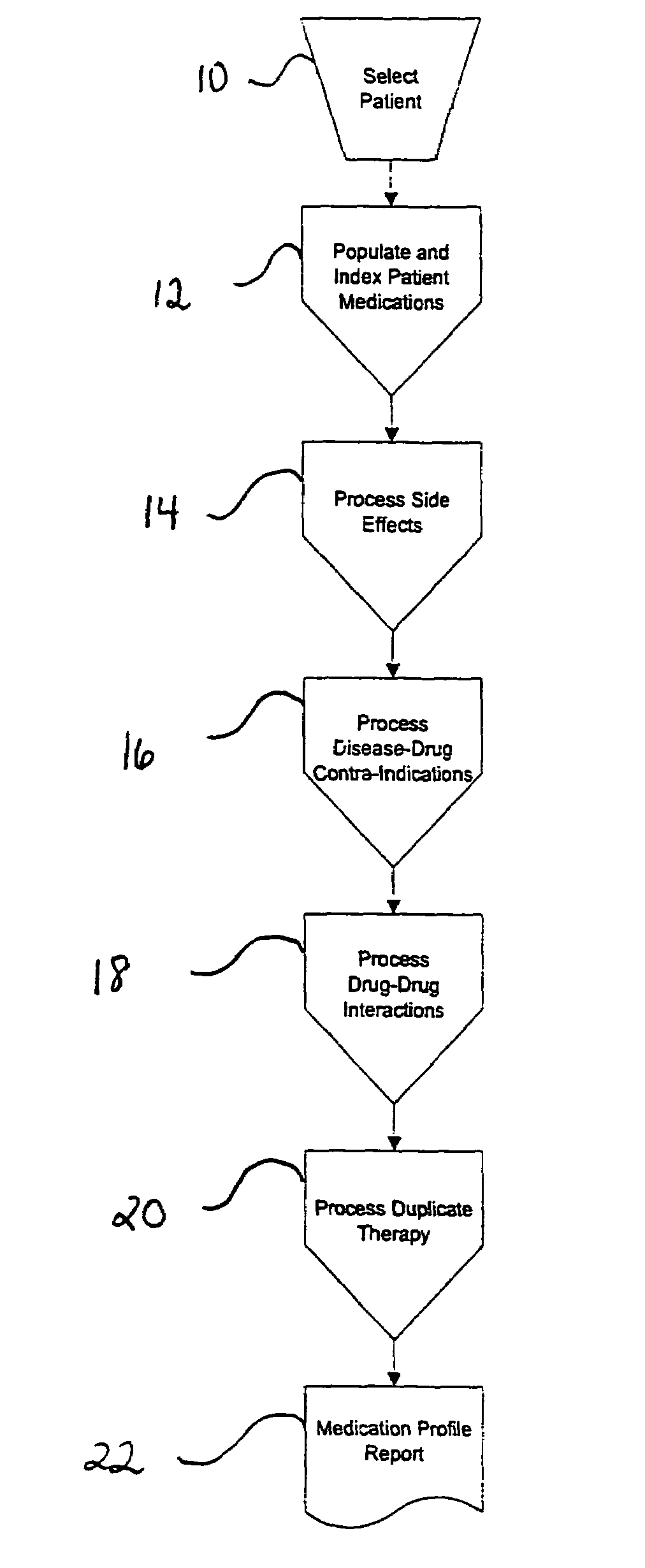
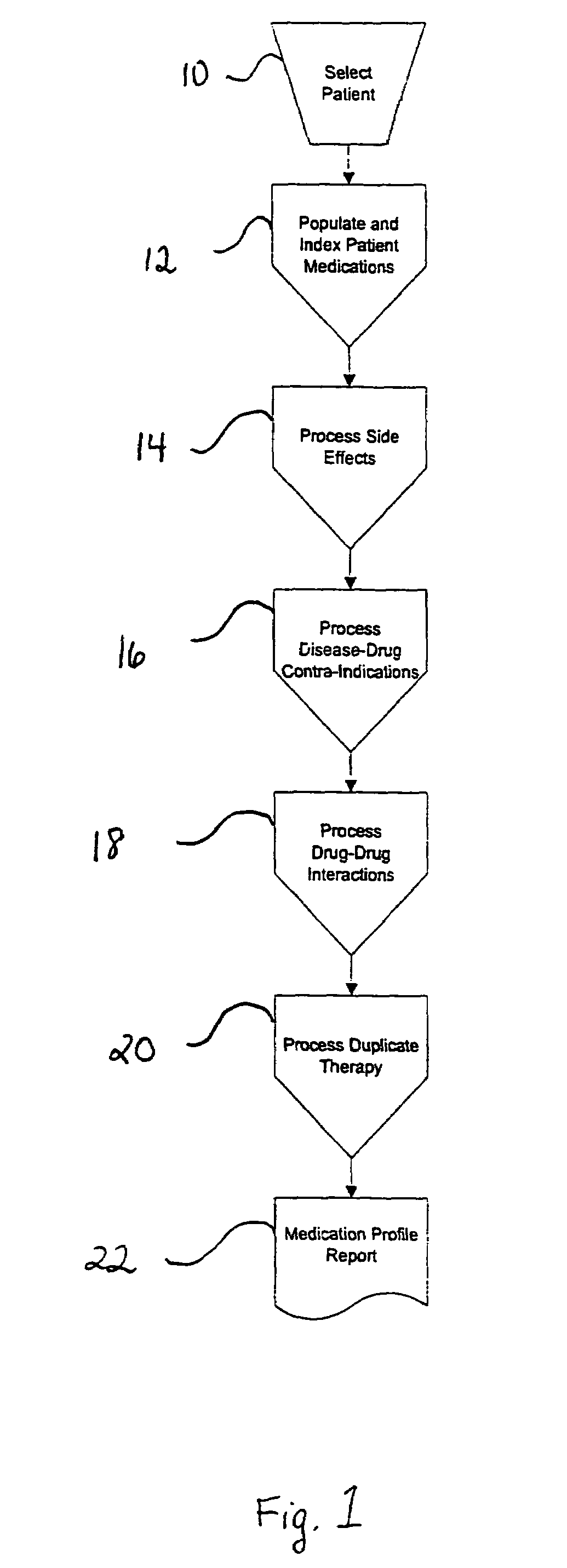
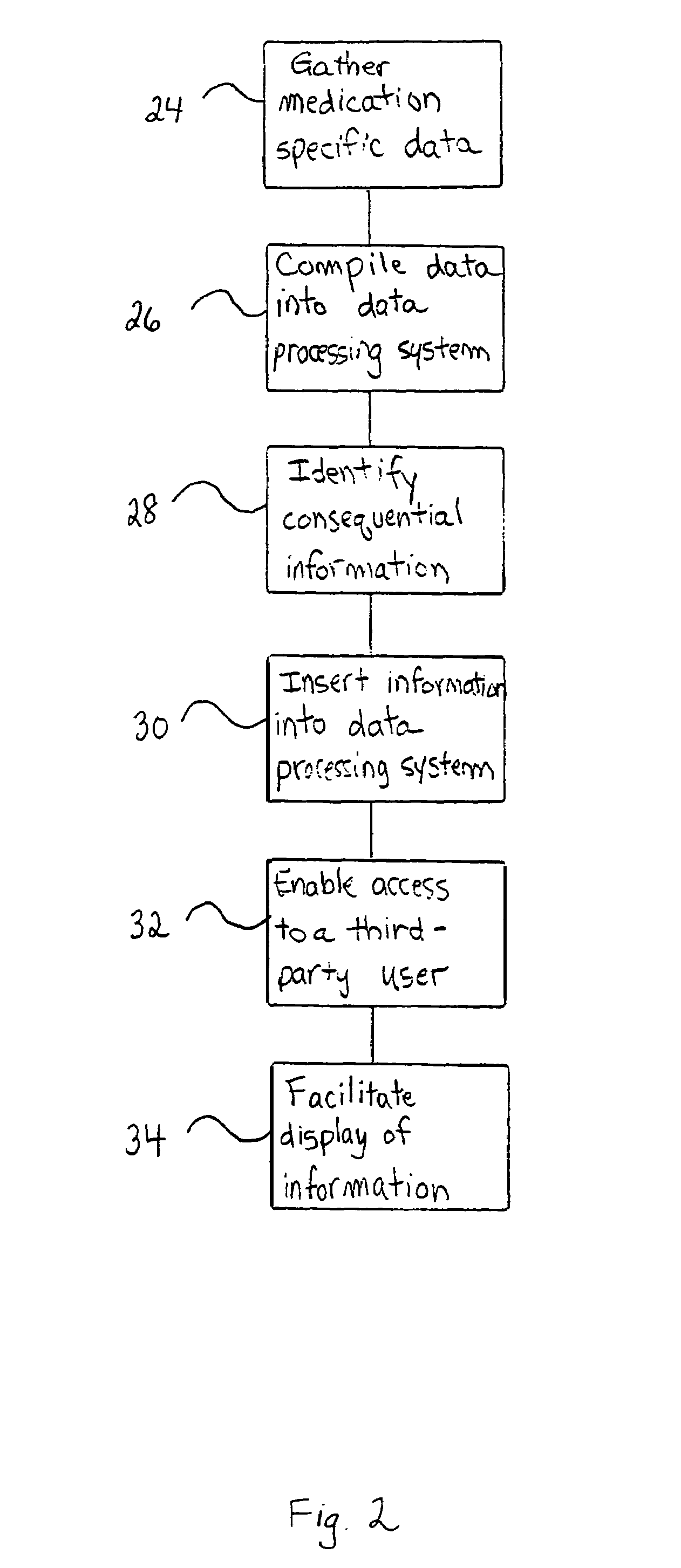
Method of generating and maintaining a patient medication profile
ActiveUS7716065B1Inhibition effectAvoid interactionData processing applicationsDrug and medicationsMedication informationDrug-drug interaction
The present invention discloses methods for conveniently providing a complete medication profile of a patient. A medication profile report may be obtained on-line by the patient or a registered provider. In addition to information regarding the expiration of prescriptions, the patient's compliance with the prescriber's directions for usage, and the names of medications being used by a patient, the medication profile report also provides the therapeutic classes of each medication, possible drug-drug interactions, and possible side effects. Convenient access to such complete medication information is highly desirable to the patient and his or her provider.
Owner:MAX WELL MEDICAL +1
Predicting drug-drug interactions based on clinical side effects
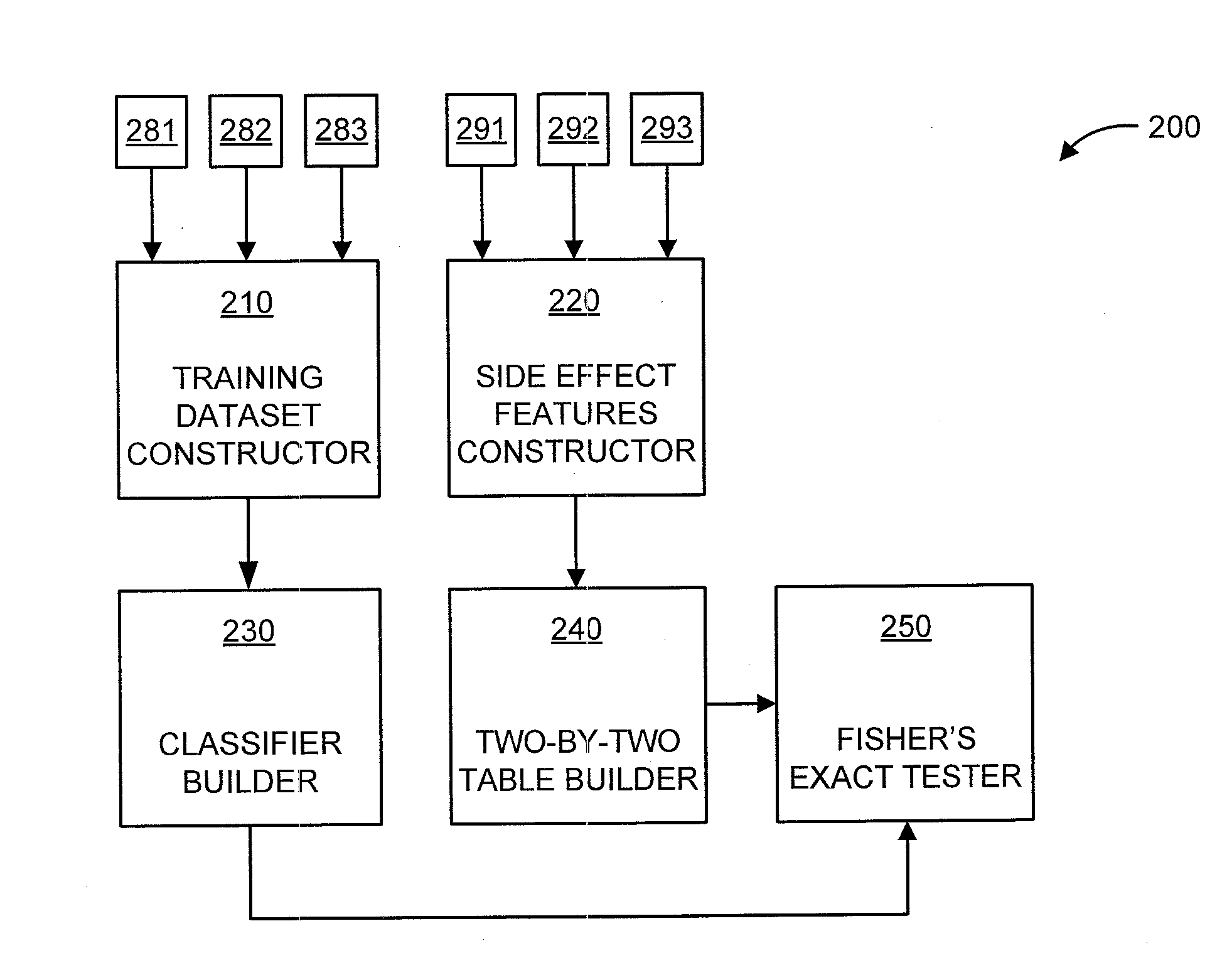
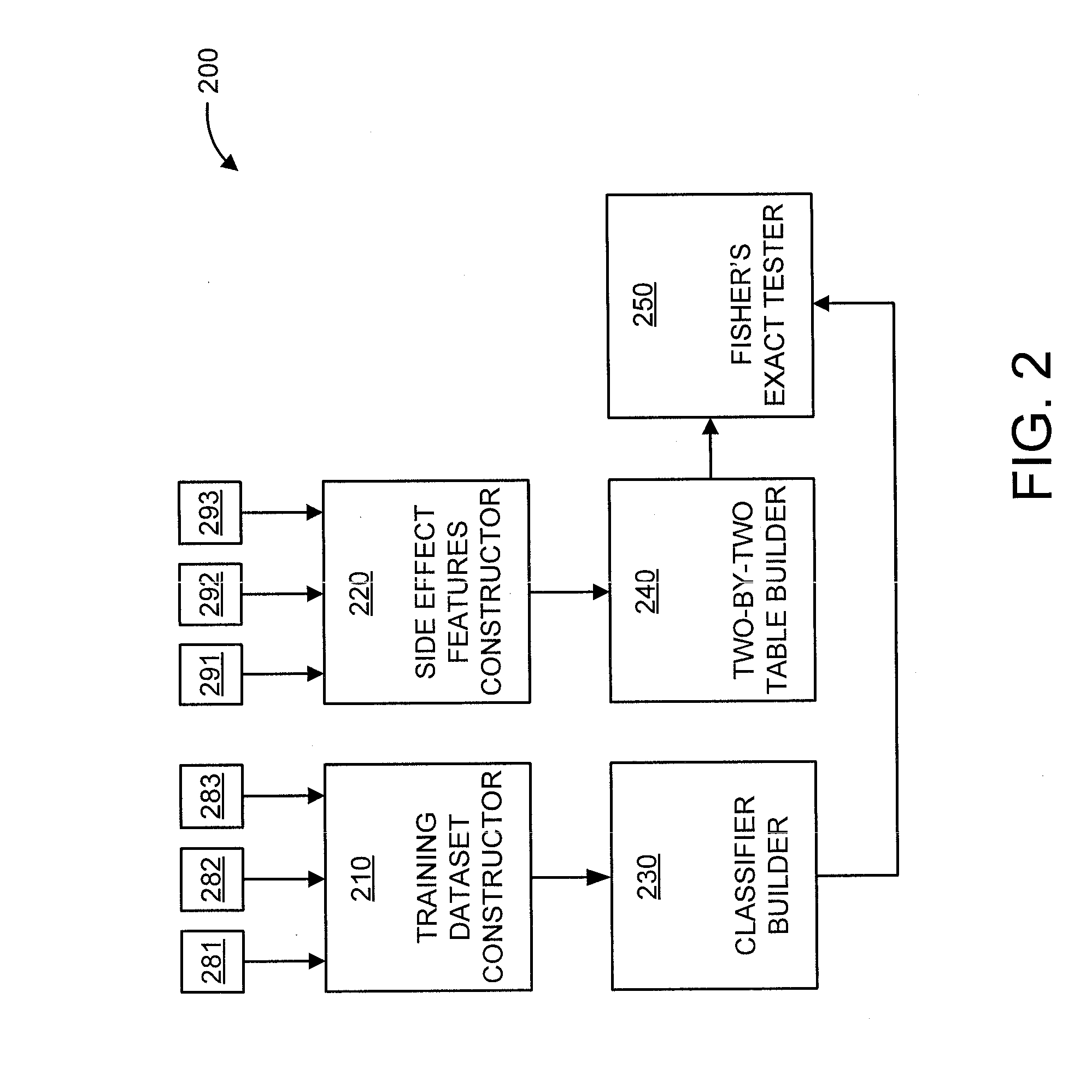
A processor-implemented method, computer program product and system are provided for predicting drug-drug interactions based on clinical side effects. The method includes constructing a drug-drug interactions training dataset that includes pharmaceutical, pharmacokinetic or pharmacodynamics drug-drug interactions from multiple data sources for each of a plurality of drugs. The method also includes constructing side effect features for each of the drugs from side effects associated with the drugs. The method further includes building, using the drug-drug interactions training dataset, a drug-drug interactions classifier that predicts adverse drug-drug interactions for drug pairs derivable from the drugs. The method additionally includes for each of the side effects, building a two-by-two table using the side effect features, and performing a Fisher's exact test using the two-by-two table to determine whether a given one of side effects is differentially shown between positive predicted drug-drug interactions and negative predicted drug-drug interactions.
Owner:IBM CORP
Compositions for reducing risk of adverse events caused by drug-drug interactions
InactiveUS20120232066A1Reduce releaseReduce actionBiocideNervous disorderDrug interactionDrug-drug interaction
The present disclosure provides a composition comprising a GABAA agonist and a GI enzyme inhibitor. The present disclosure also provides a composition comprising (a) a GI enzyme inhibitor and (b) a first drug that interacts with a second drug to produce an adverse effect when the second drug is co-ingested as a GI enzyme-cleavable prodrug with the first drug. Such an interaction can be additive or synergistic.
Owner:SIGNATURE THERAPEUTICS
Method for acquiring and analyzing a list of a patient's prescription medications
InactiveUS20060136272A1Improper prescriptionWide accessDrug and medicationsComputer-assisted medical data acquisitionCaregiver personDosing regimen
A computer system for obtaining, analyzing and providing information to a community of user patients regarding their medication is disclosed. The system is provided by means of world wide web access and generates a user patient screen prompting the manual entry of data relating to the use patient and drugs being taken. The data are analyzed and results are provided to the user patient and / or the caregiver including drug-drug interactions, drug pricing, alternative medications and possible adjustments in the dosing regimen of the user patient.
Owner:RUBSAMEN REID M
Stable pharmaceutical composition for atherosclerosis
ActiveUS20120027849A1Good antihypertensive effectGood blood pressure effectBiocideAntipyreticLipid formationLipid lowering drug
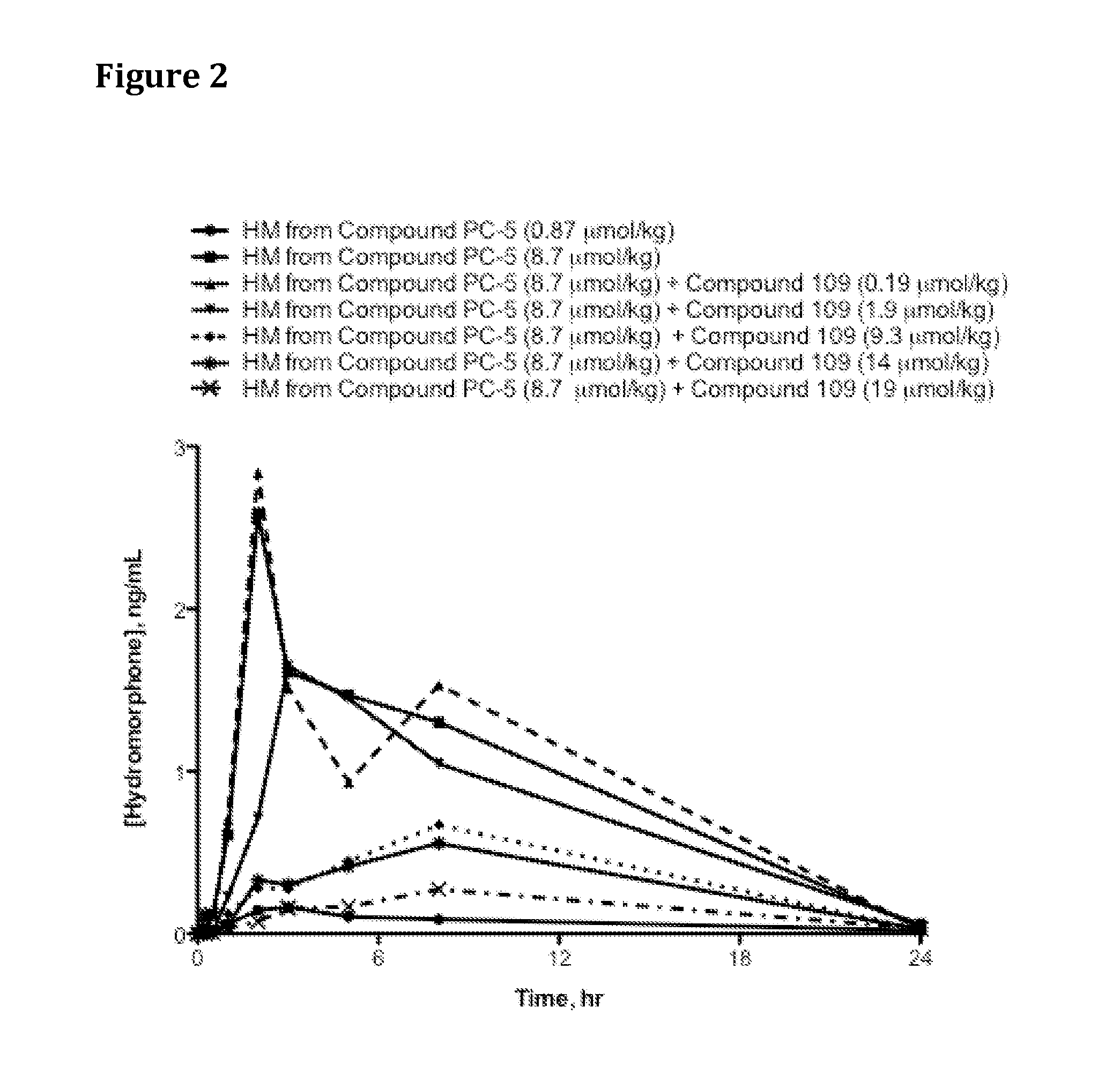
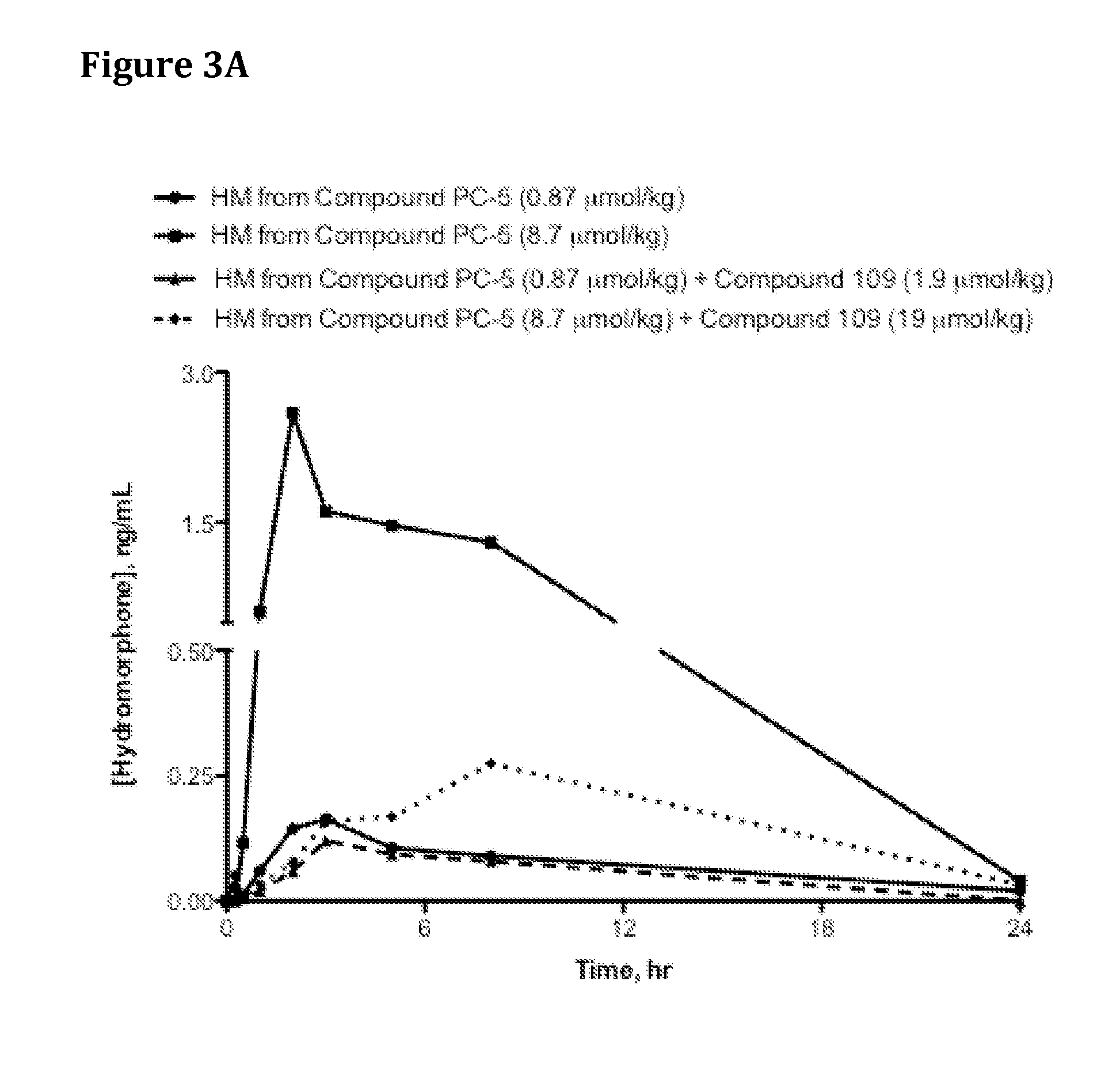
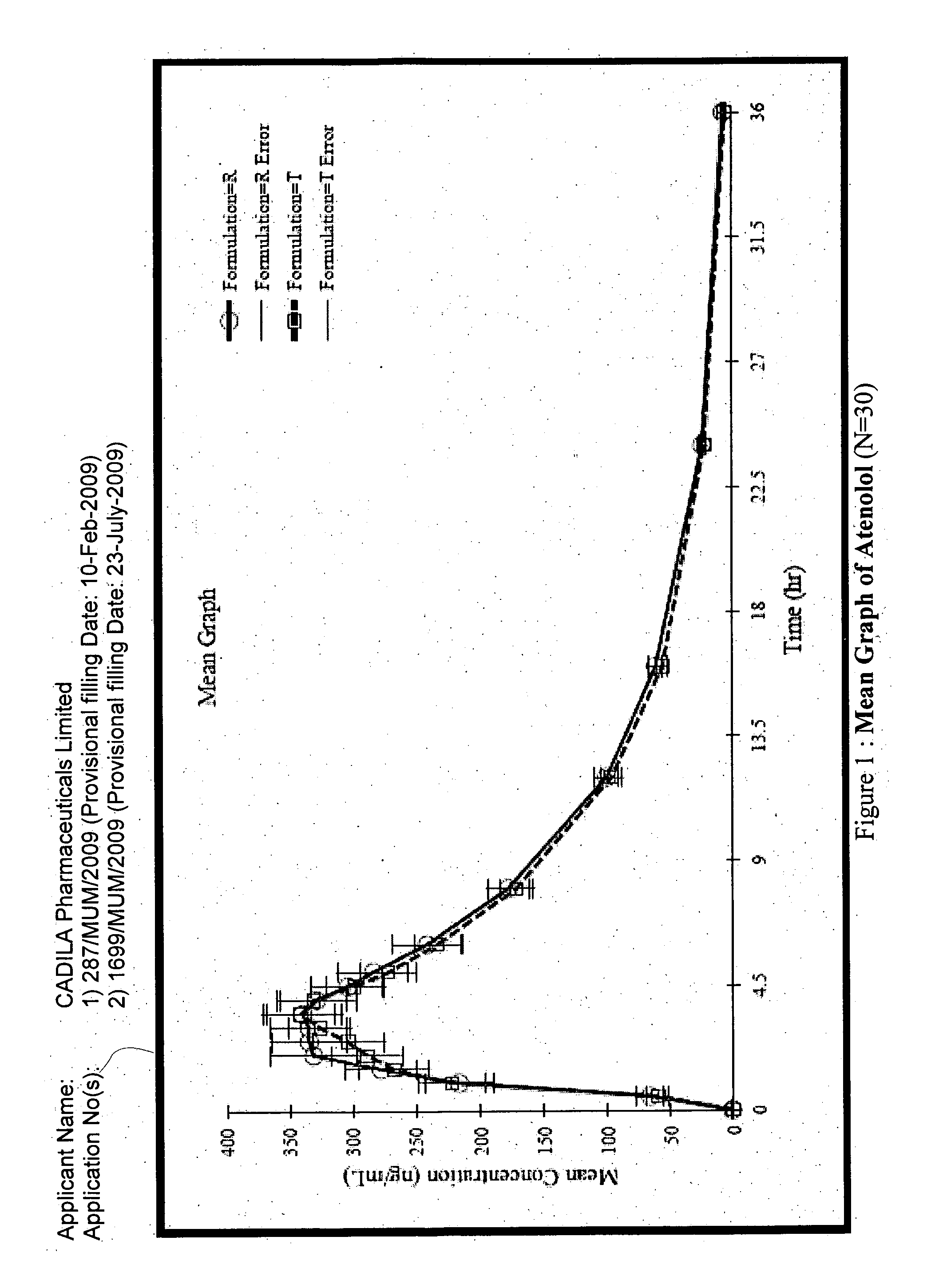
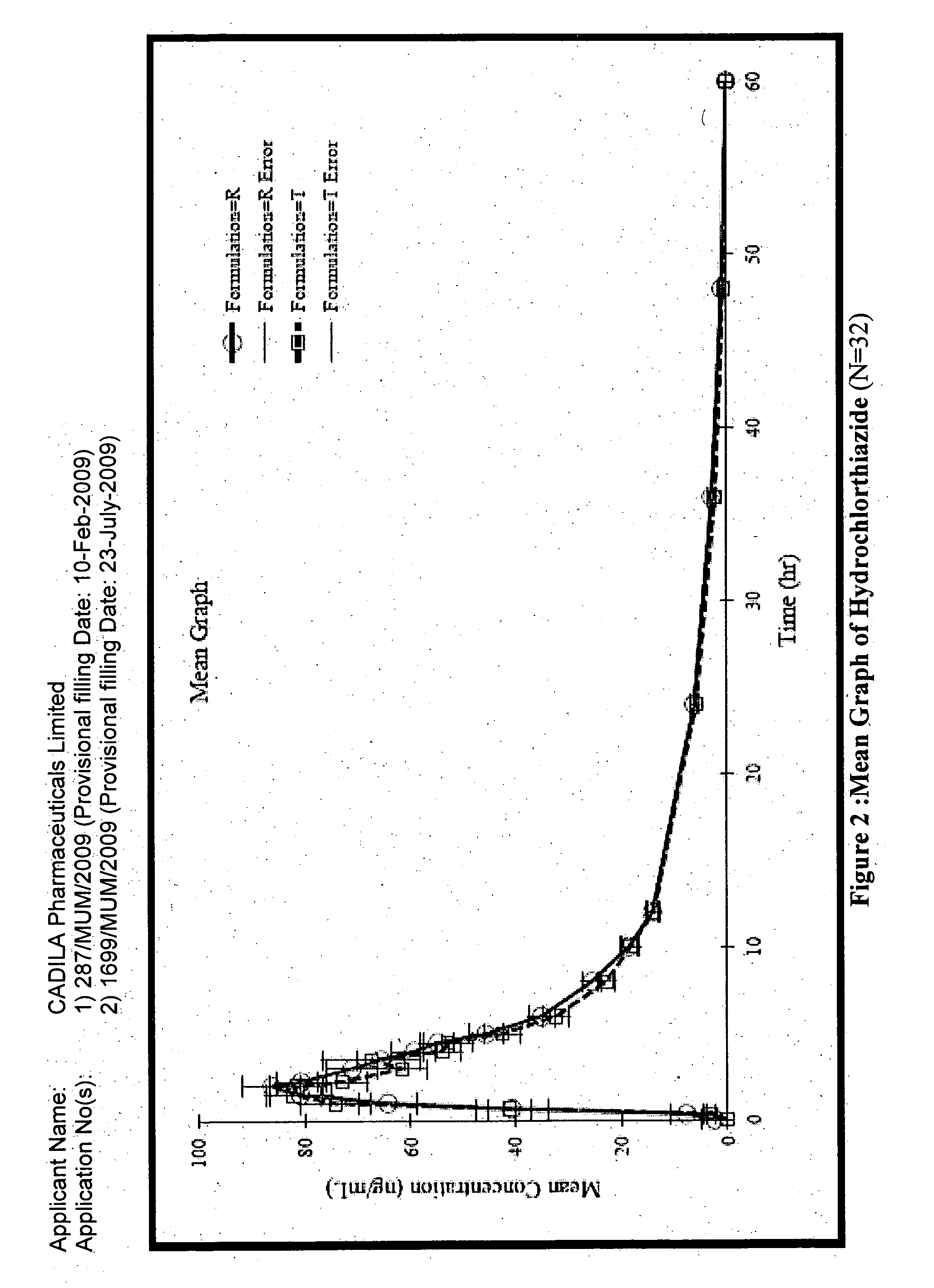
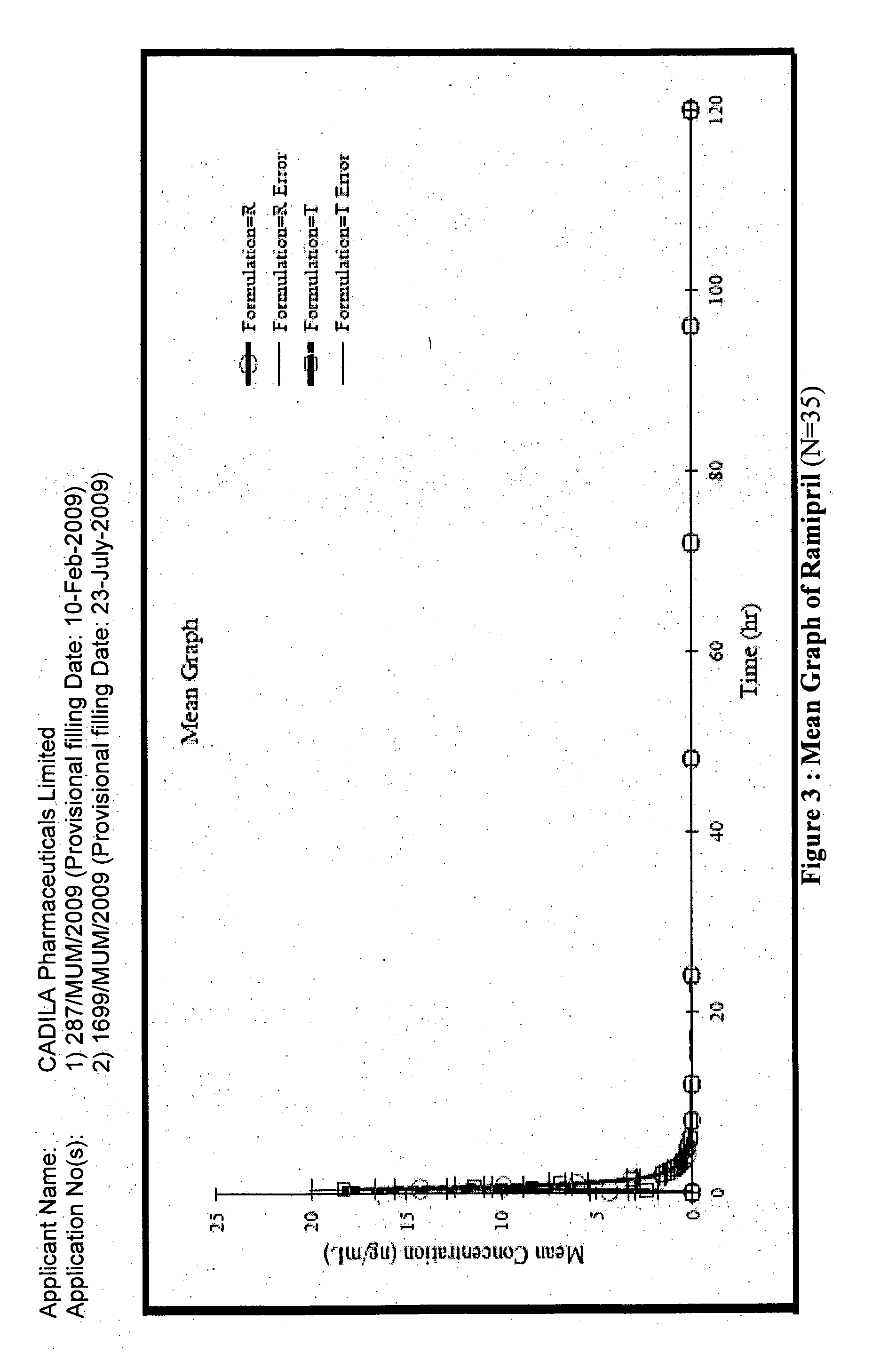
The present invention relates to a stable solid oral pharmaceutical multi-component composition comprising combination of blood pressure lowering drugs with lipid lowering agent / s and optionally a platelet aggregation inhibitor in a single dosage form. The blood pressure lowering agents are selected from β-adrenergic receptor blocking agent, ACE inhibitor and diuretic. The lipid lowering agent is selected from HMG Co-enzyme-A reductase inhibitor. The pharmaceutical composition made as per present invention a) overcomes any drug-drug interactions, b) exhibits pharmacokinetic and pharmacodynamic profile of individual therapeutic agent, c) has minimal side effects. The invention provides multi-component composition (MCC) to increase adherences to therapy. The MCC as per present invention provides compositions that maintain activity of all active ingredients without significant increase in adverse event profile. The present invention further relates to a method of preparing the said pharmaceutical composition.
Owner:CADILA PHARMA
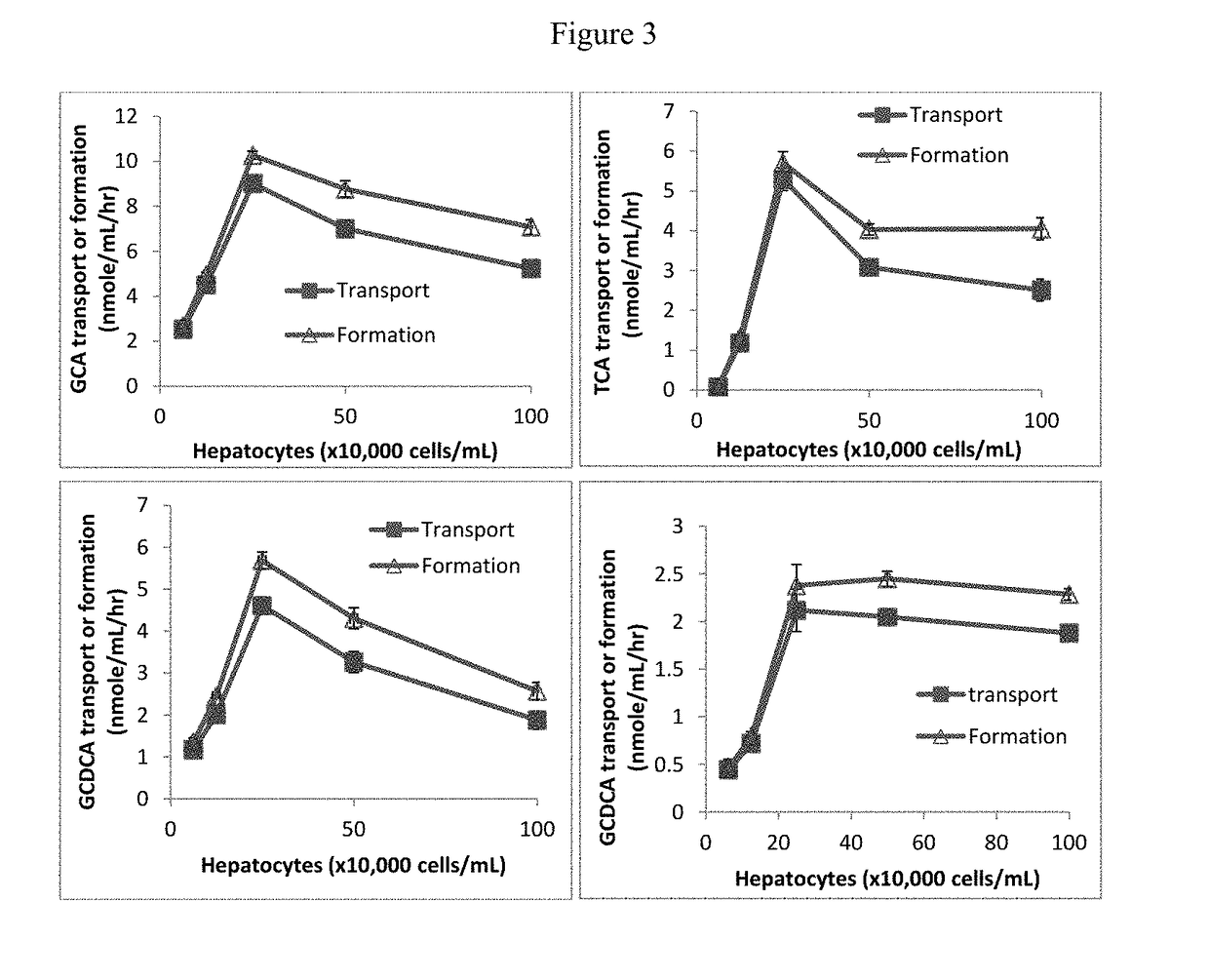
Method for measuring bile salt export transport and/or formation activity
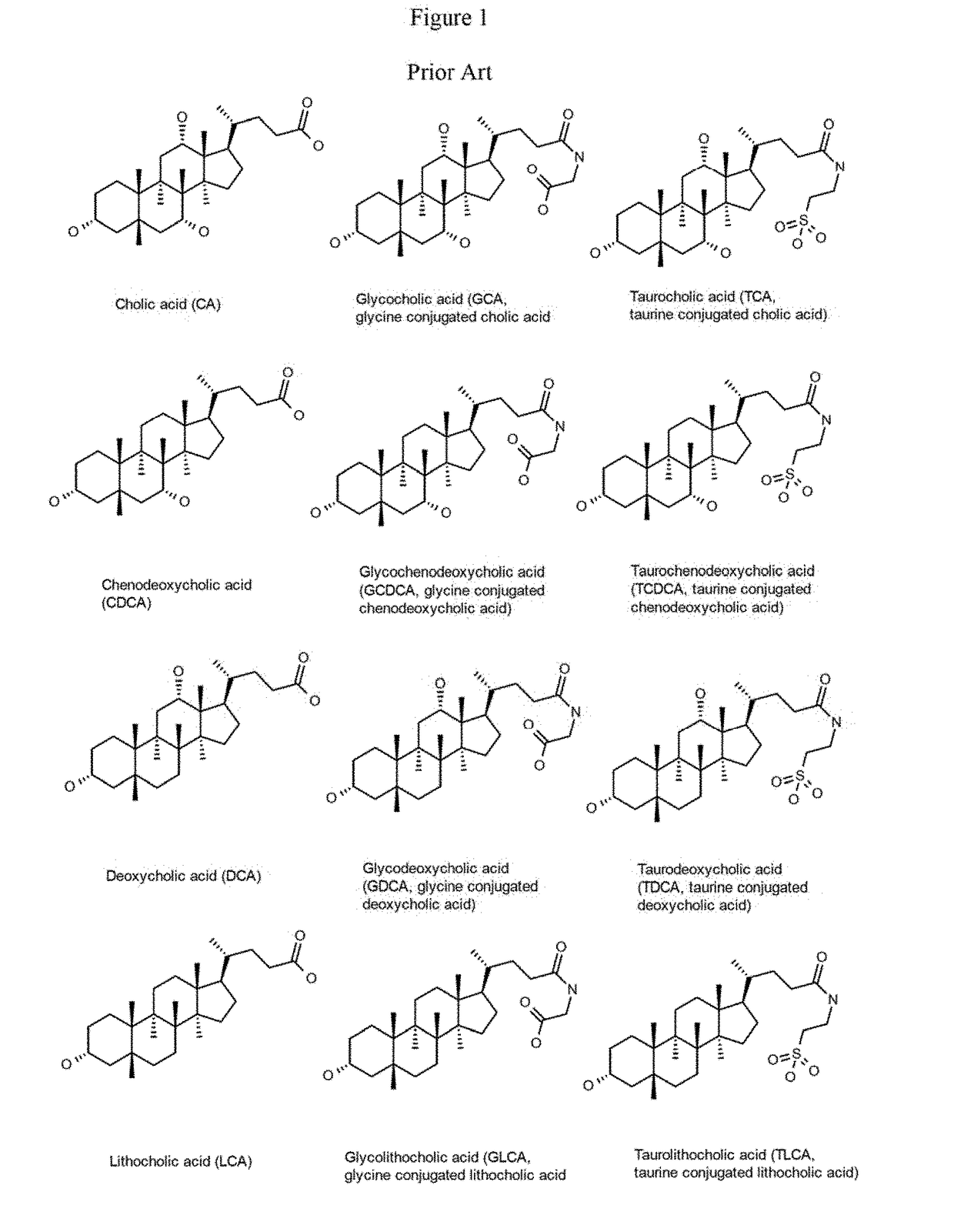
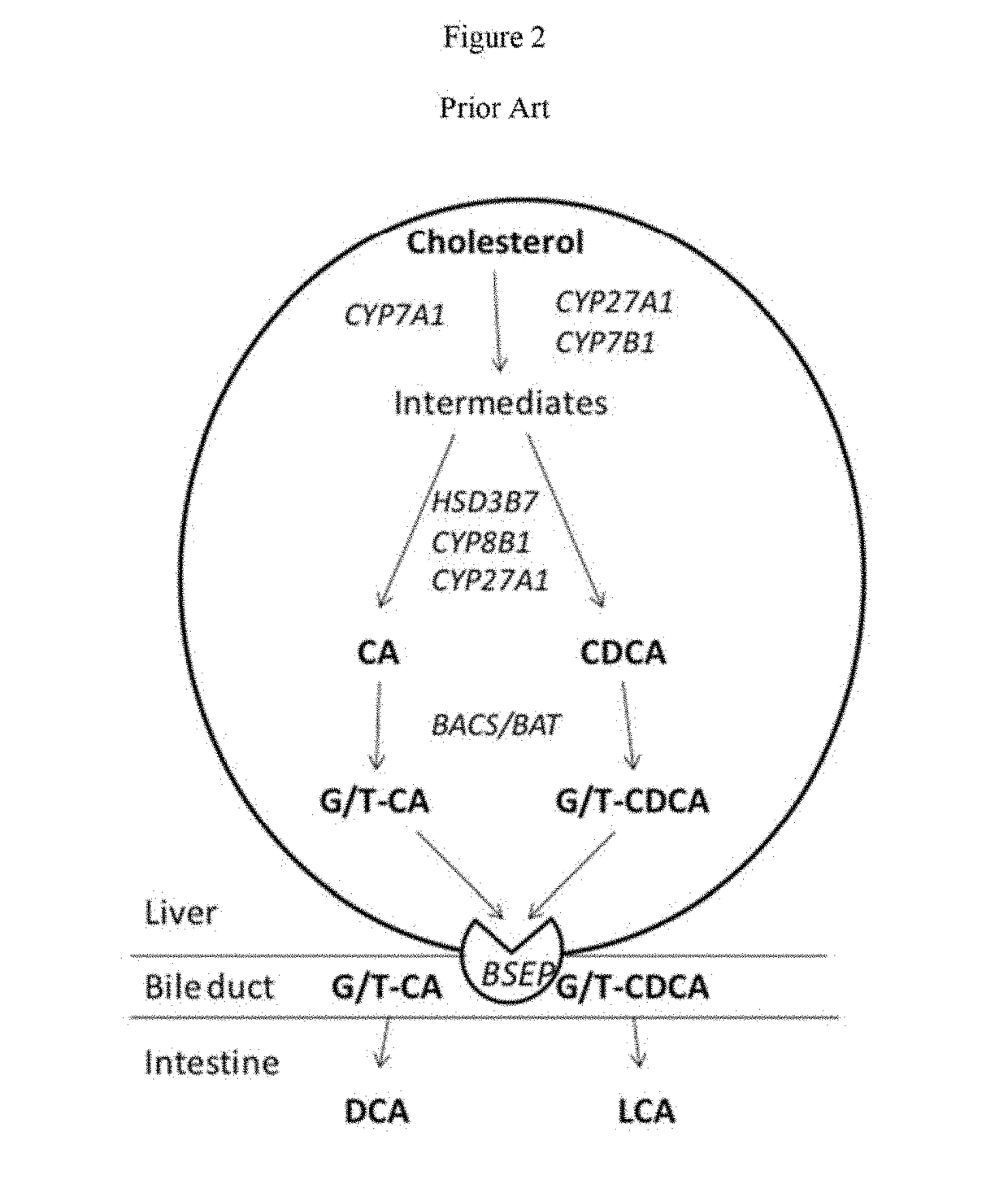
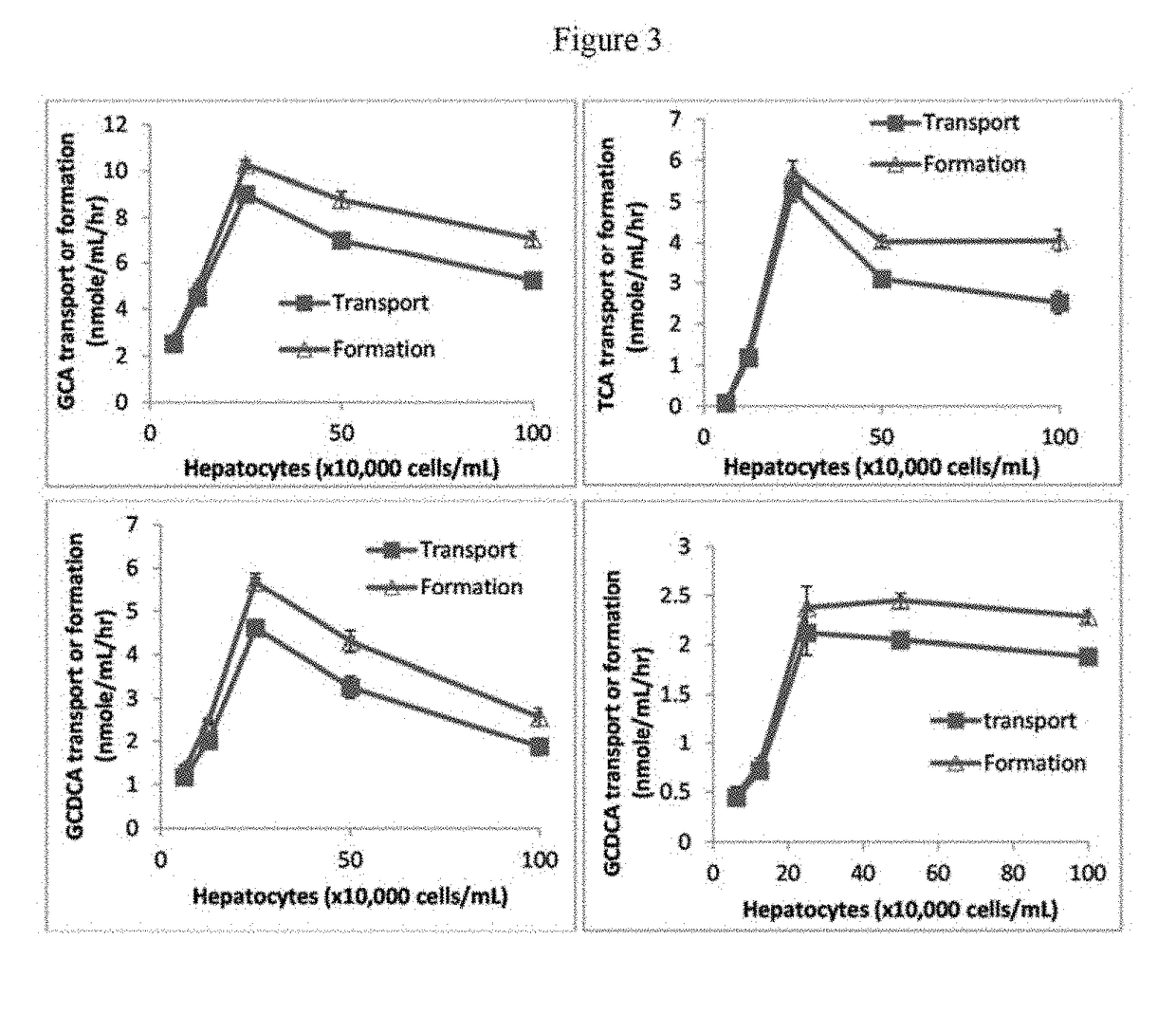
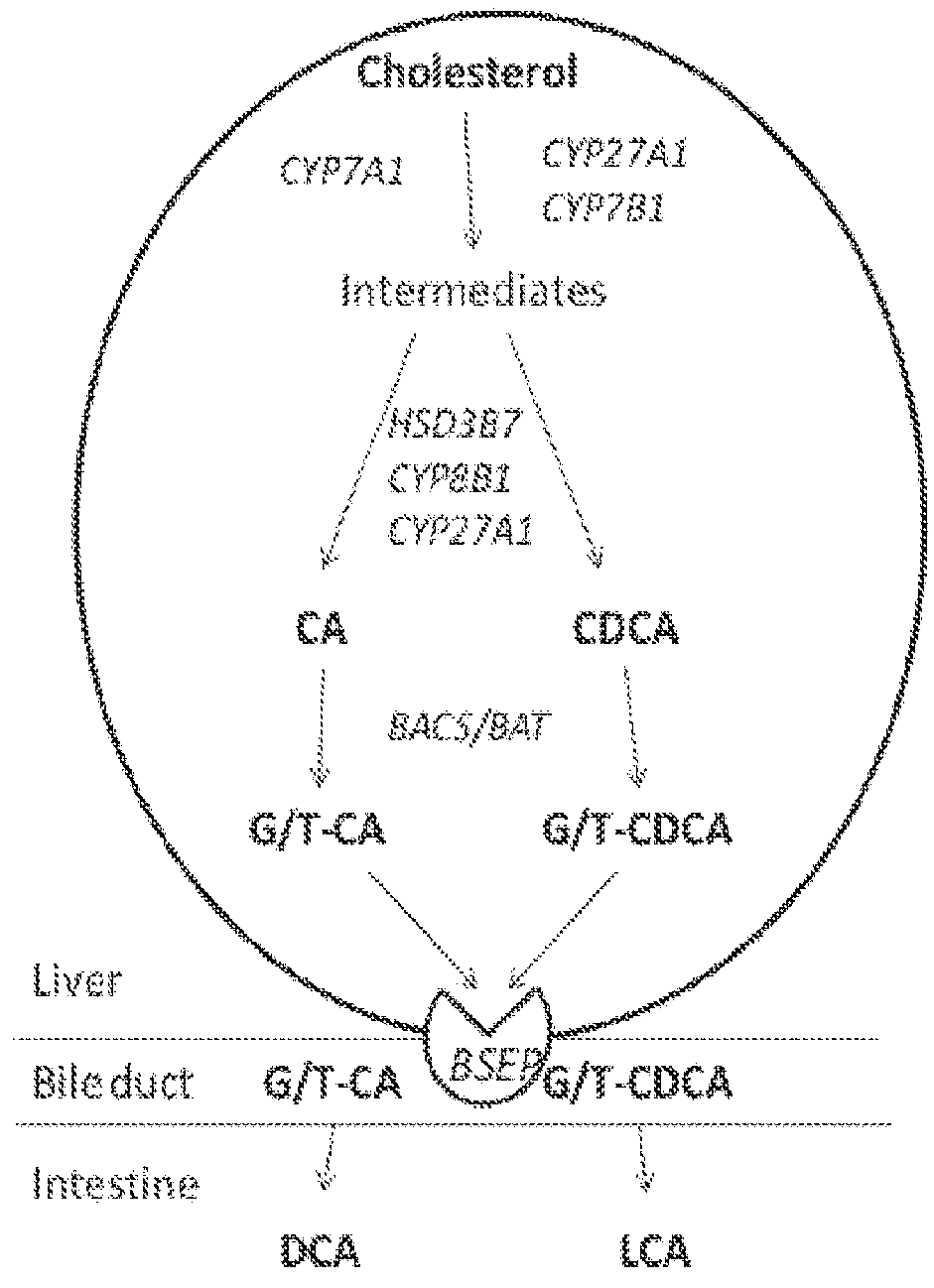
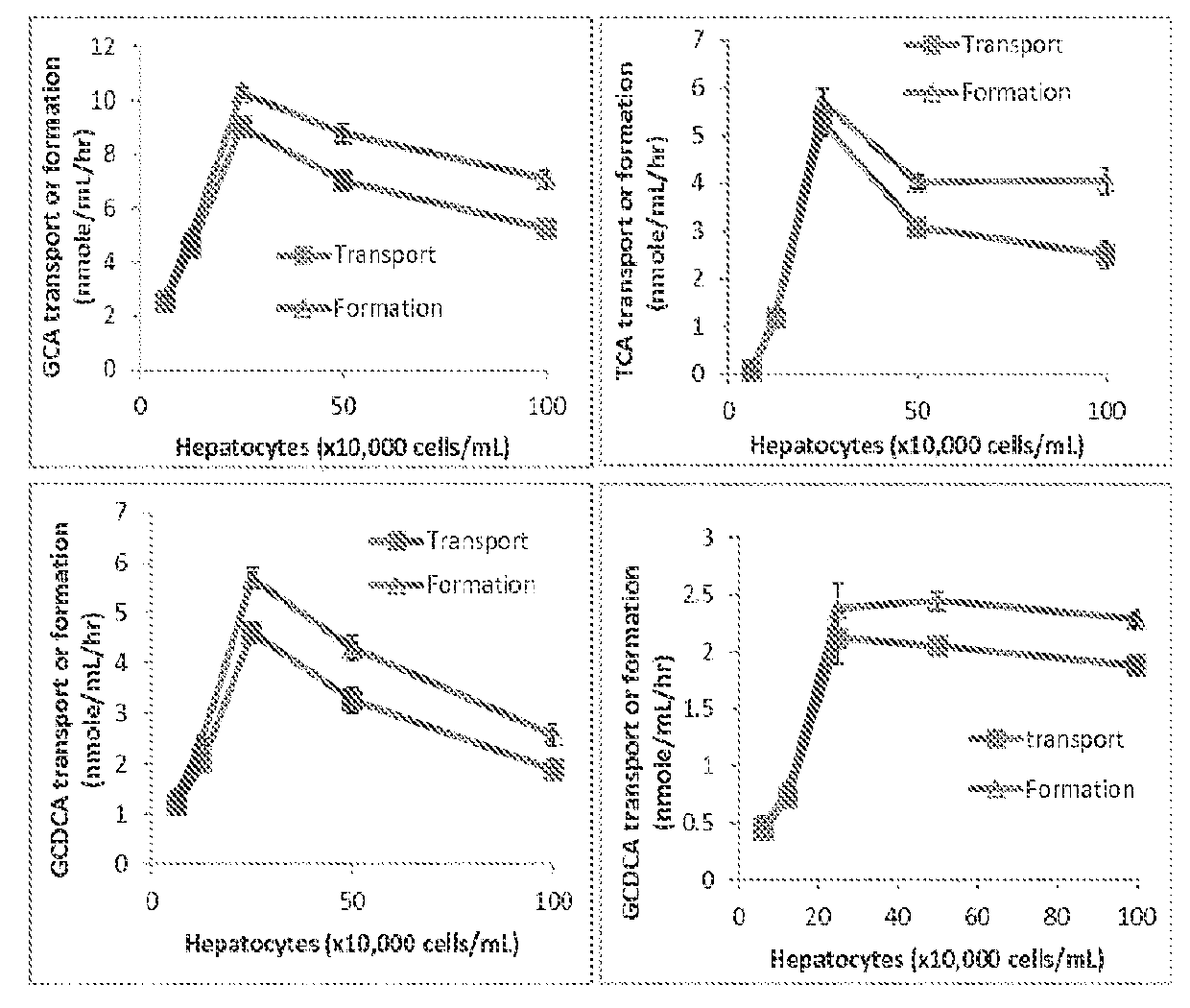
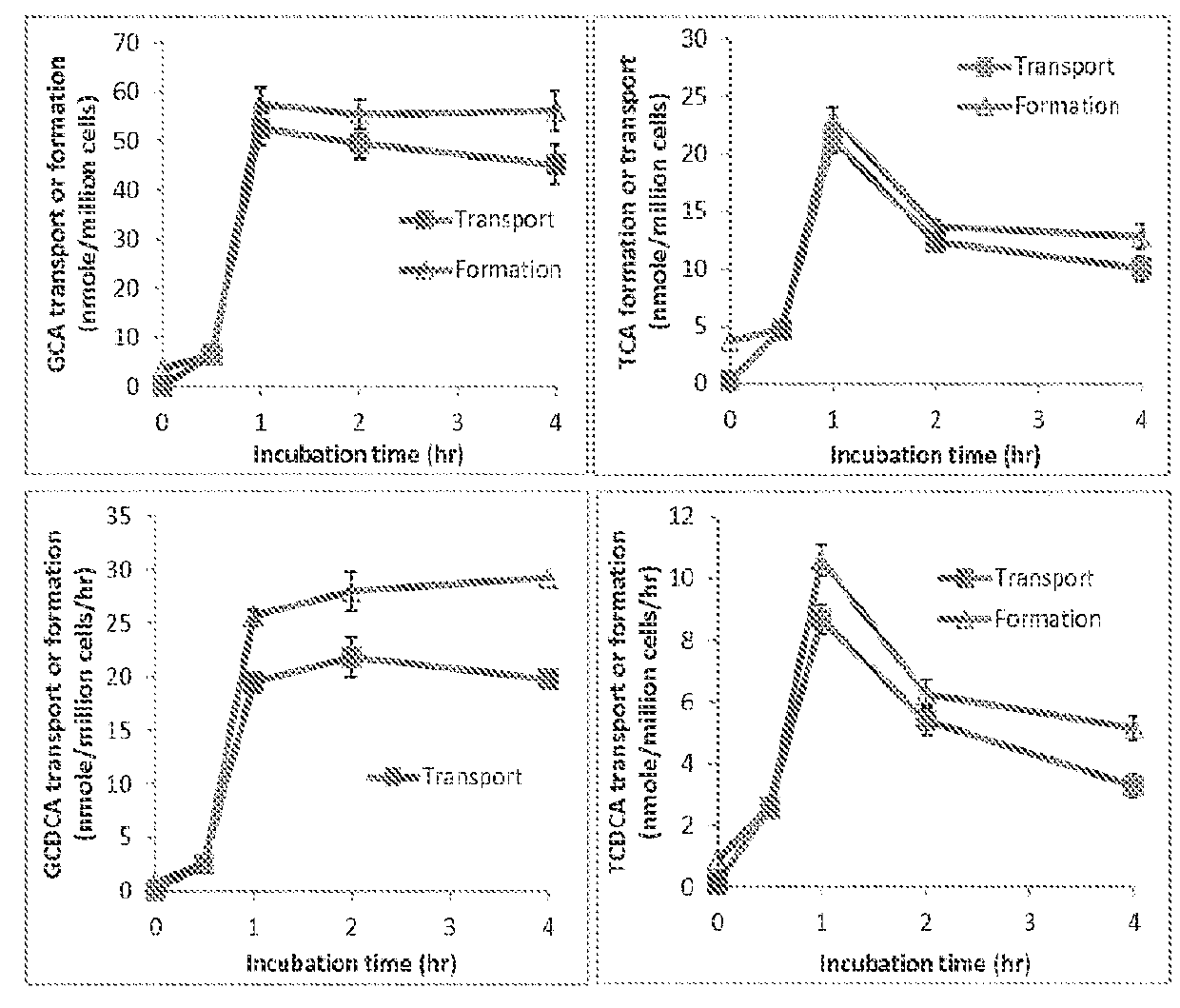
A method is provided to measure modulation of bile salt export transport and / or formation activity in hepatocyte or stable cell line preparations by test agents including but not limited to drugs, drug candidates, biologicals, food components, herb or plant components, proteins, peptides, DNA, RNA. Furthermore, the method is to determine modulation of bile salt export transport and / or formation activity not only by said test agents, but further their metabolites or biotransformed products formed in situ. The bile salt export transport and / or formation activity modulation includes but not limited to inhibition, induction, activation and / or regulation. The method can be practiced to identify test agents, which have potential to cause liver injury, drug-drug interactions, and / or can be used as therapeutic agents for the treatment of cholestasis, abnormality of bile salt metabolism, liver diseases and cholesterol abnormality.
Owner:BIOTRANEX
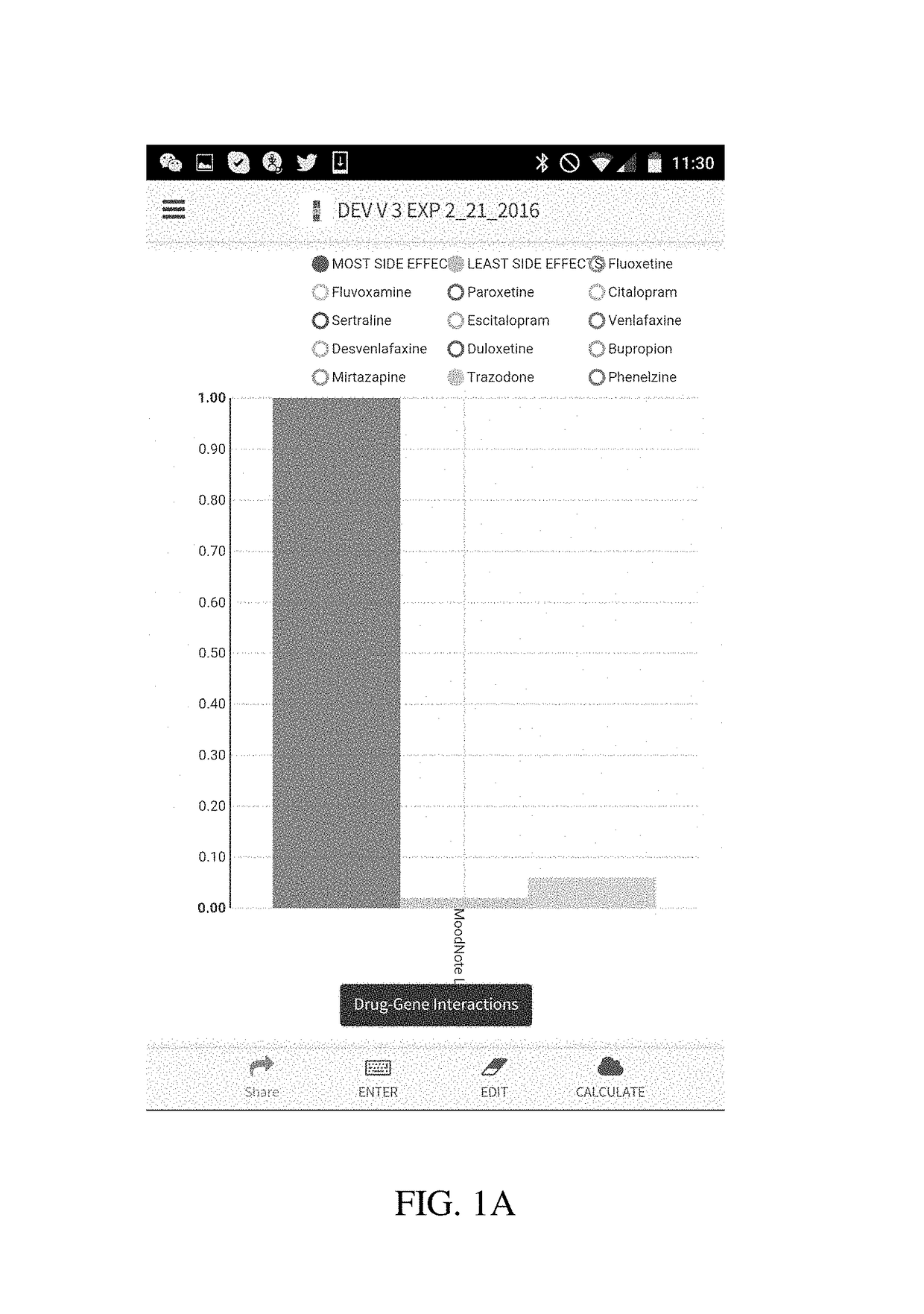
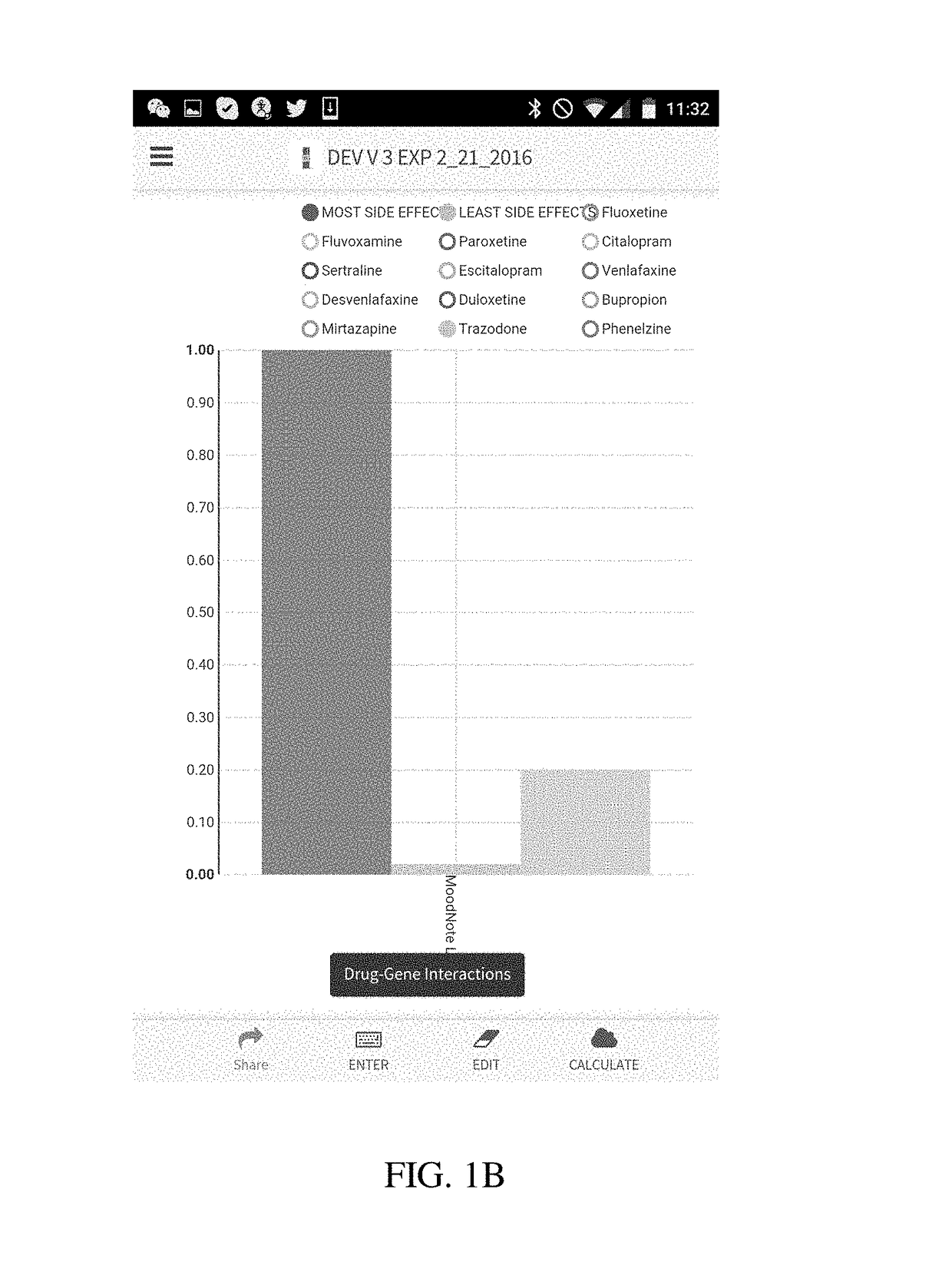
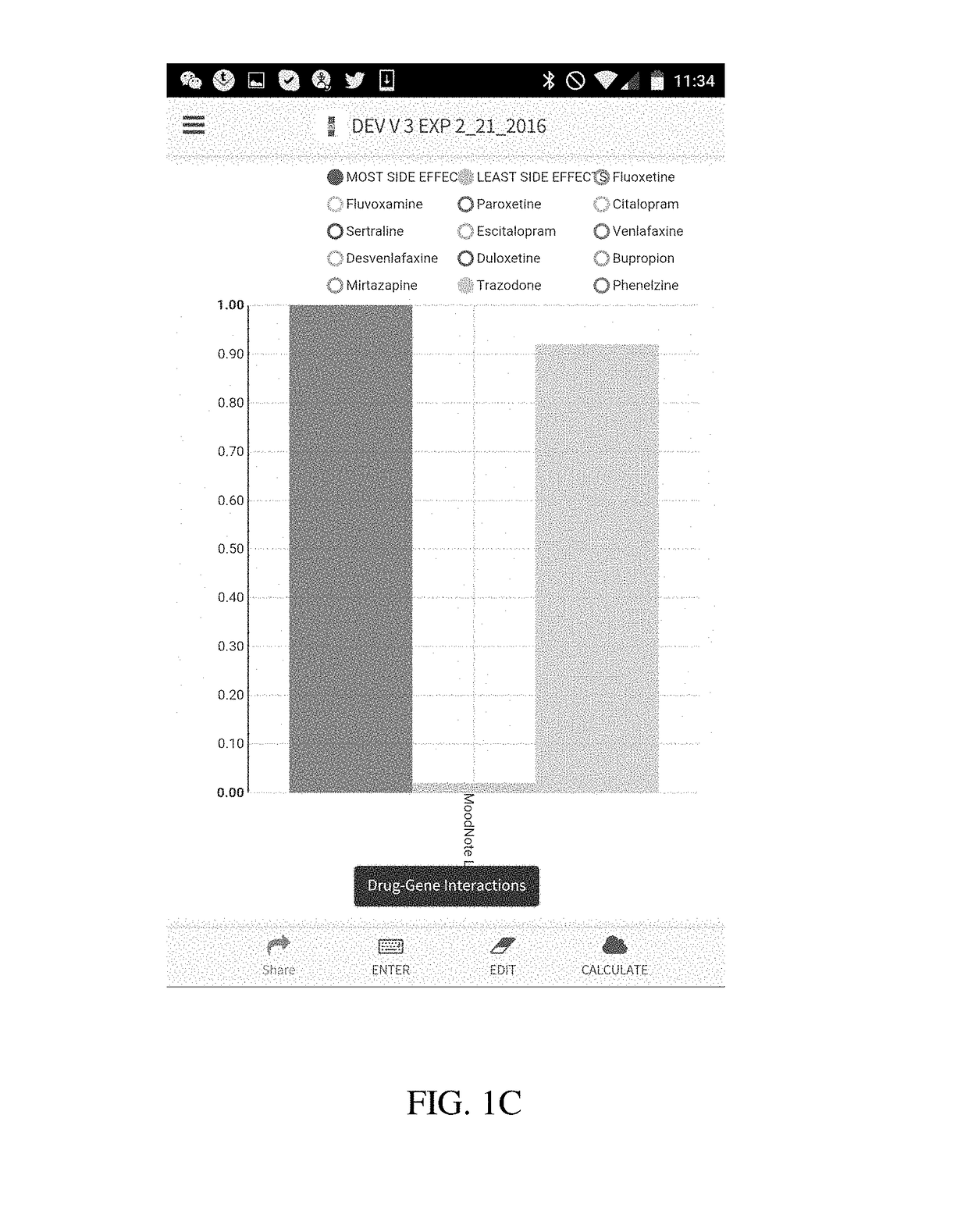
Method and system for calculation and graphical presentation of drug-drug or drug-biological process interactions on a smart phone, tablet or computer
InactiveUS20170270246A1Good choiceDrug and medicationsData visualisationVisual presentationTablet computer
A method and system is provided for visualization and pictorial presentation to a user of possible interactions between a prospective drug that is being considered for prescribing to a person and that person's genotype. Genetic information of the person that can affect the manner in which a drug acts on a molecular, physiological or biological function of the body or a tissue, or a manner in which a drug is being metabolized, absorbed, excreted or otherwise eliminated from the body or a tissue by the body or tissue systems, is entered into a computerized device. The computerized device conducts a search of a drug database for drugs that have known interactions with the entered genetic information, and assigns a numeric value to each of a plurality of drugs, either in aggregate, as a class, or individually, in order to quantify the nature, strength and direction of each interaction. The computer sends the assigned numeric values to the computer's output module for their visual presentation to a user as a graph including a panel of columns, or other geometrical structures, whose geometrical characteristics correspond to the assigned numeric values of each drug, in order to facilitate the prospective drug selection by a prescriber on the basis of the totality of drug-gene and / or drug-drug interactions presented to the user as a visual graph.
Owner:BASKYS ANDRIUS
Application of two-photon fluorescence probe for detecting cytochrome oxidase CYP3A4
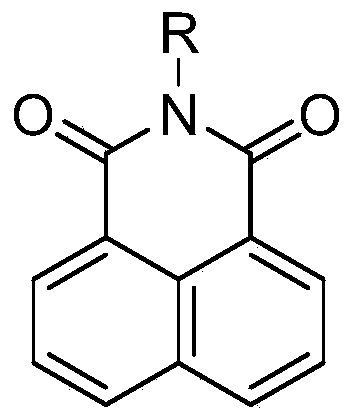
ActiveCN109928927AEasy to detectHigh selectivityOrganic chemistryFluorescence/phosphorescenceMetaboliteIn vivo
The invention discloses application of a two-photon fluorescence probe for detecting cytochrome oxidase CYP3A4, and belongs to the technical field of biomedicine. The specific probe substrate can be used for measuring the enzyme activity of the CYP3A4 in a biological system. The procedure for determining CYP3A4 enzyme activity is as follows: Naphthalimide 4-position hydroxylation is selected as aprobe reaction, and the CYP3A4 enzyme activity in various biological samples can be determined by quantitatively detecting the amount of hydroxylated metabolites generated per unit time. The two-photon fluorescence probe can be used for quantitative evaluation of the CYP3A4 enzyme activity in the biological samples of different species and different individual sources, and quantitative determination of CYP3A4 activity in animal tissue cell culture fluids and cell preparations of different sources so as to realize evaluation of drug disposal capability of the important drug metabolizing enzymeCYP3A4. The two-photon fluorescence probe can also be used to rapidly screen inhibitors of the CYP3A4 in vitro, evaluate inhibitory ability of the inhibitors and detect the CYP3A4 activity in tumors,can detect the CYP3A4 activity in zebrafish, and can be used to detect drug-drug interaction of the CYP3A4 in vivo.
Owner:DALIAN MEDICAL UNIVERSITY
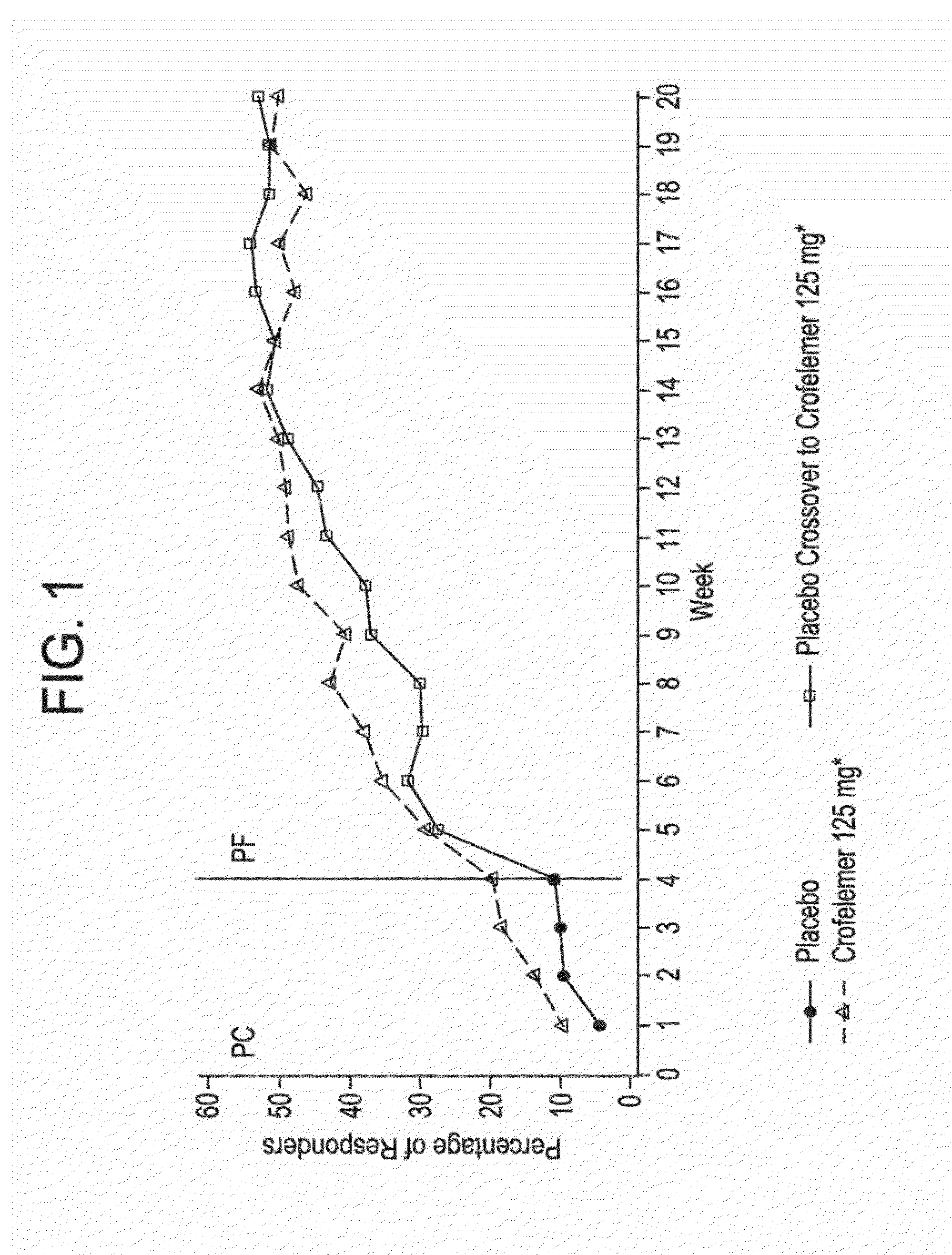
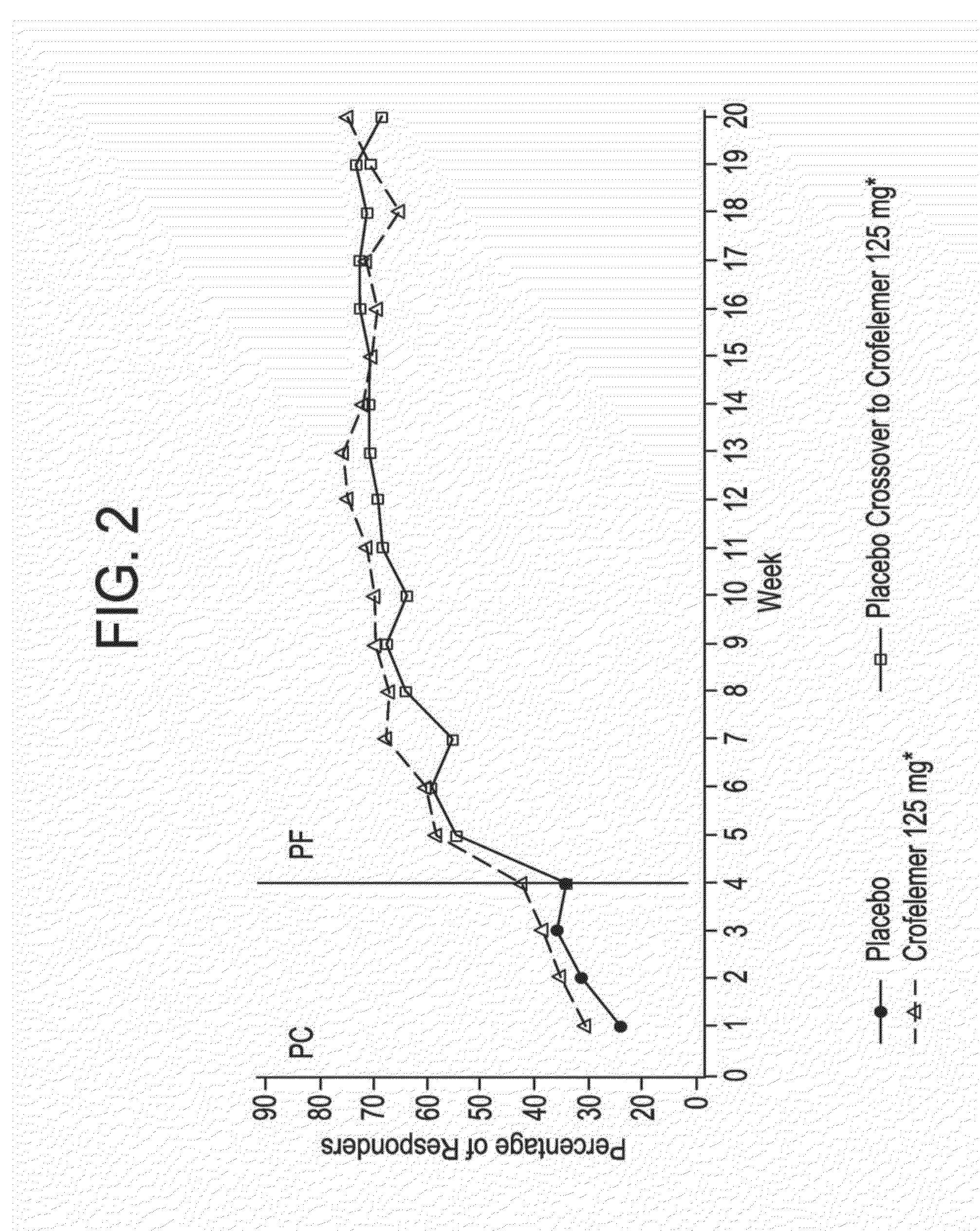
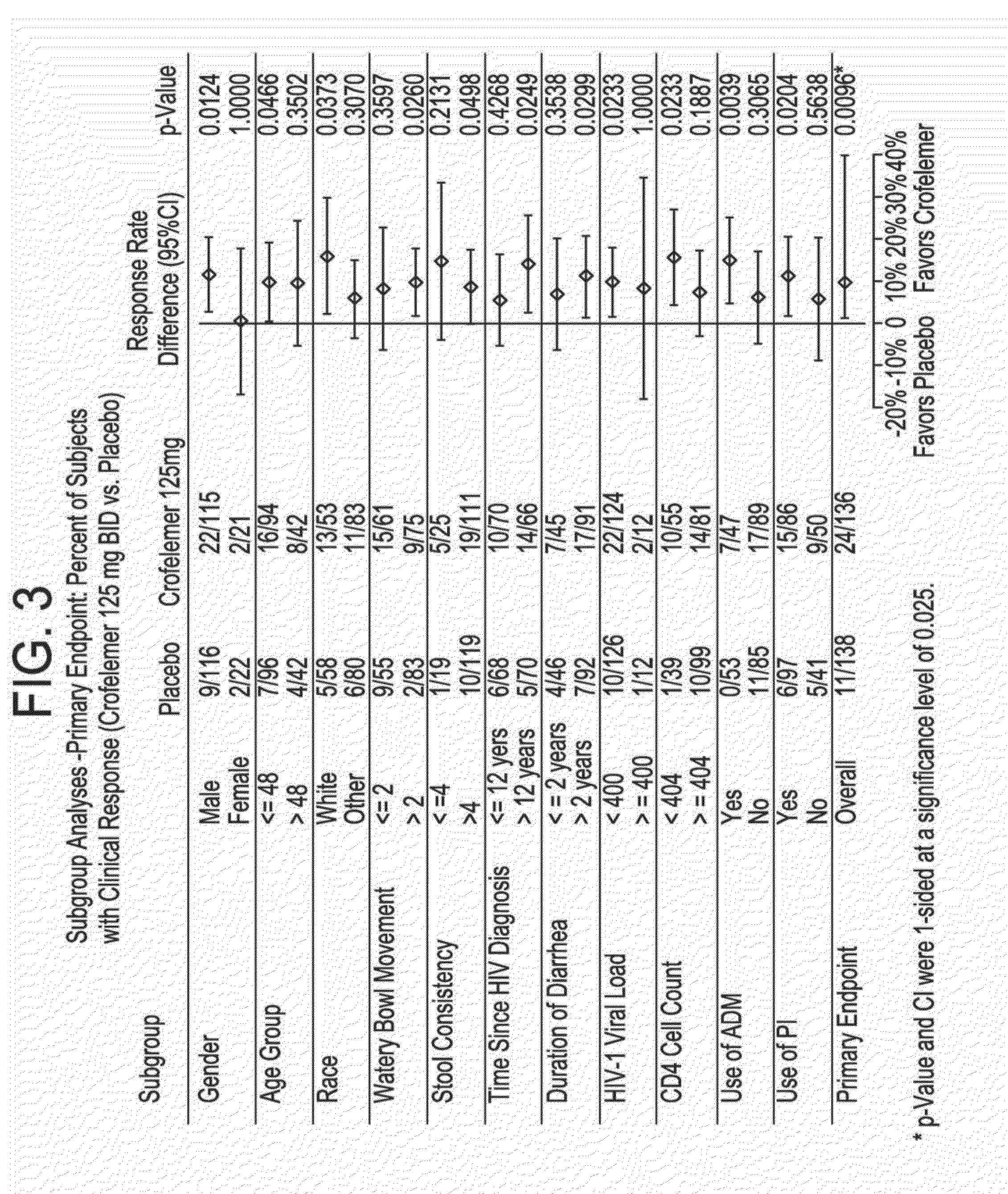
Methods and compositions for treating hiv-associated diarrhea
InactiveUS20140163096A1Not cause deterioration of immune statusBiocideDigestive systemDrug-drug interactionAntiretroviral therapy
Provided herein are methods for treating HIV-associated or highly active antiretroviral therapy (HAART)-associated diarrhea in an HIV positive subject by administering a composition comprising crofelemer to the subject wherein the composition has minimal drug-drug interactions with at least one other compound concurrently administered to the subject to treat an HIV infection. Also provided are methods for treating HIV-associated or highly active antiretroviral therapy (HAART)-associated diarrhea in an HIV positive subject by administering a composition comprising crofelemer to the subject, wherein the composition does not significantly inhibit the activity of at least one other compound concurrently administered to the subject to treat an HIV infection.
Owner:NAPO PHARMA INC
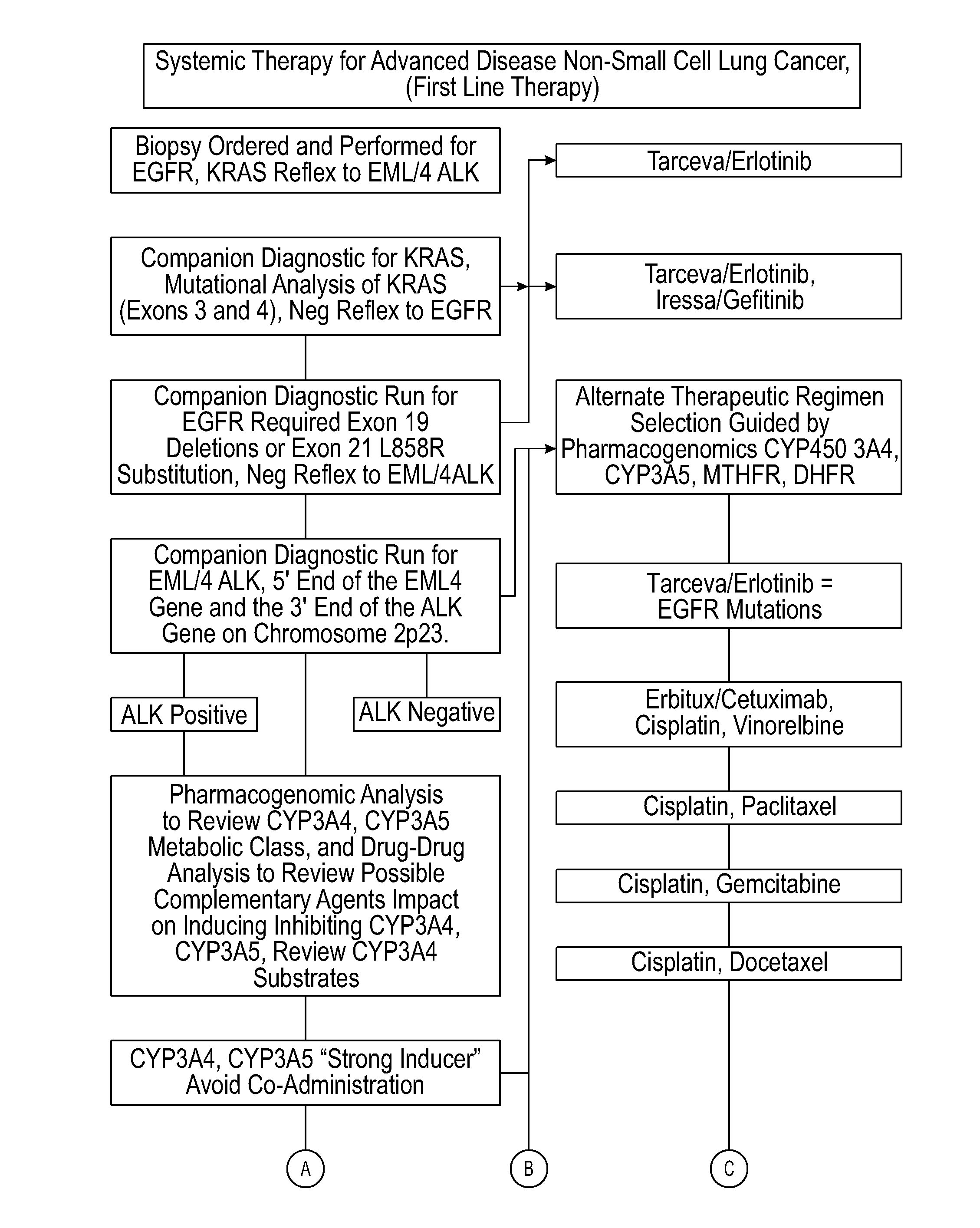
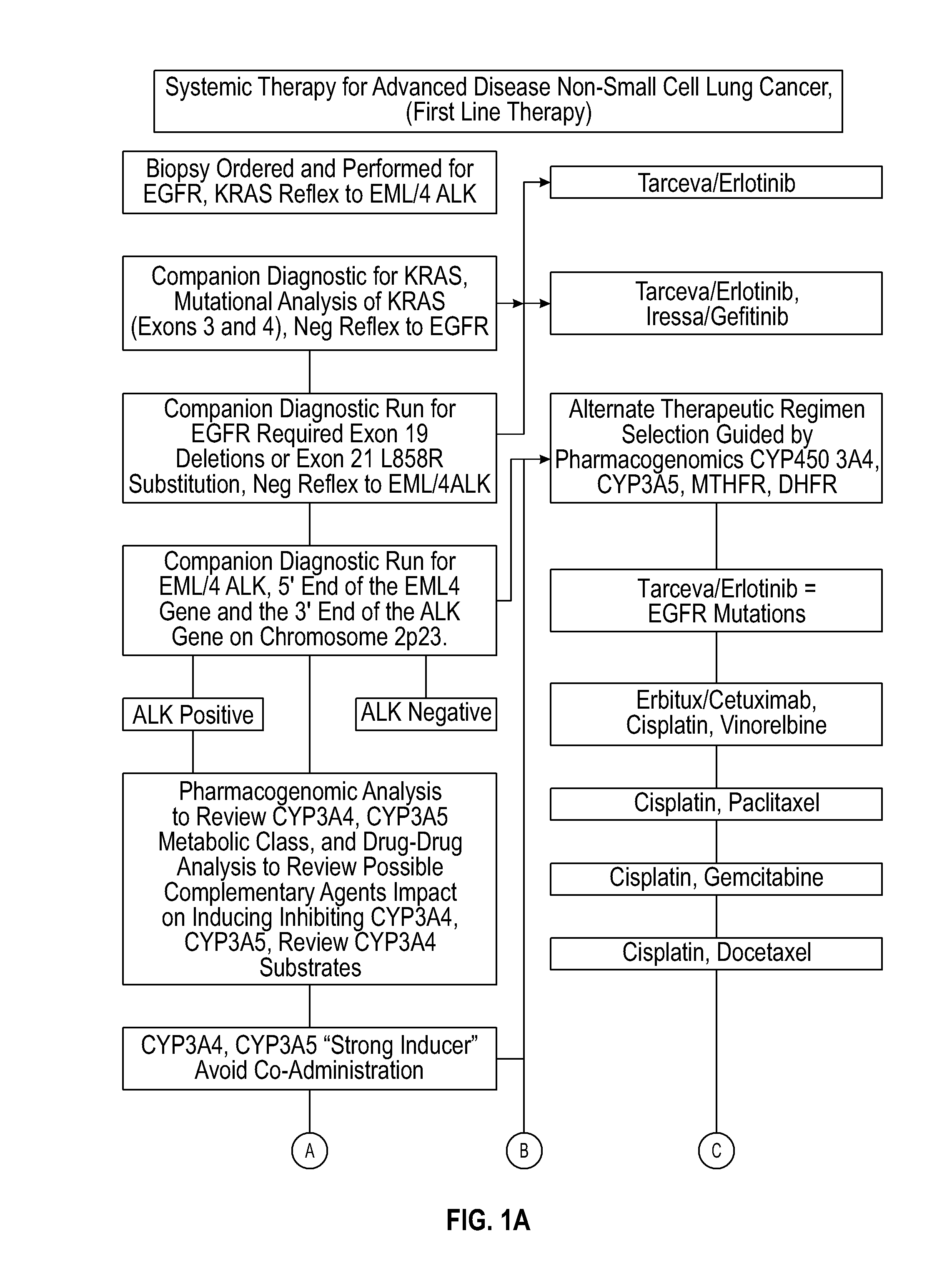
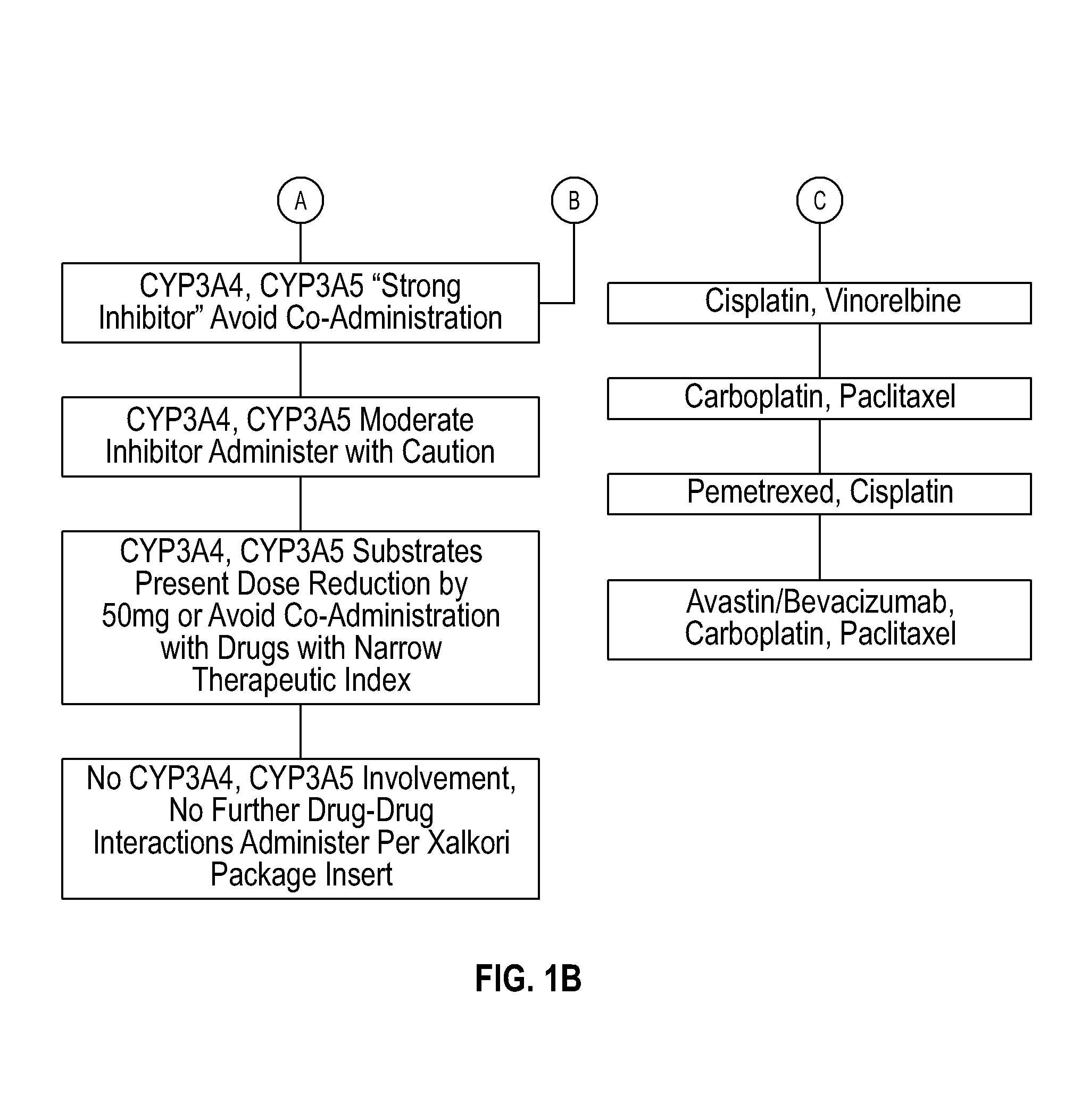
Systems and methods for optimizing drug therapies
InactiveUS20150154375A1Reduce adverse side effectsData processing applicationsDrug and medicationsPharmacogenomicsDrug-drug interaction
Several embodiments disclosed herein relate to methods of providing an optimized drug therapy that is specialized or customized for an individual subject or a group of subjects based, at least in part, on one or more of the genetic profile of the subject, the pharmacogenomic profile of the subject, and / or evaluation of possible drug-drug interactions. In several embodiments, systems specialized for performing one or more aspects of the methods are provided.
Owner:COMPANION DX REFERENCE LAB
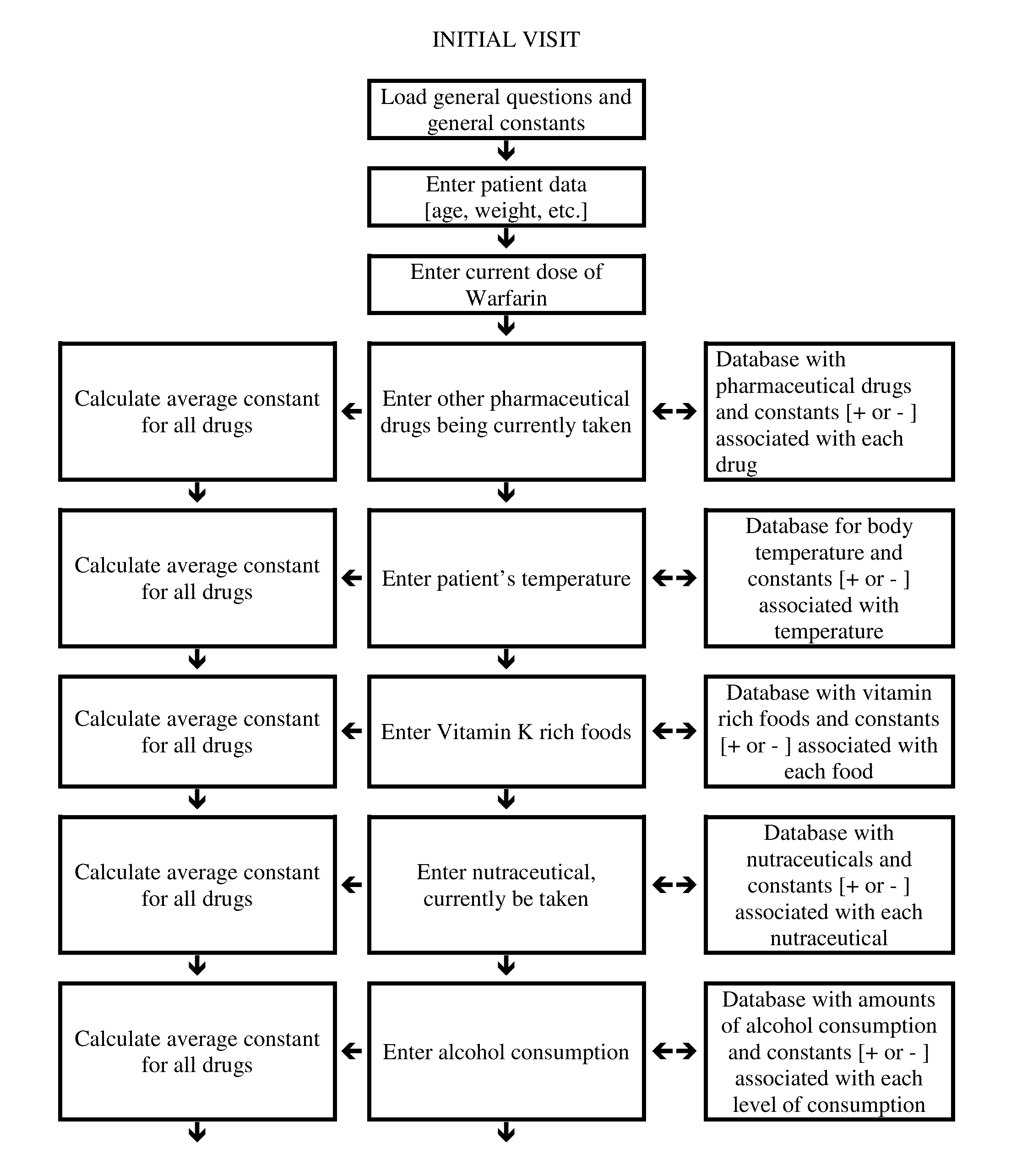
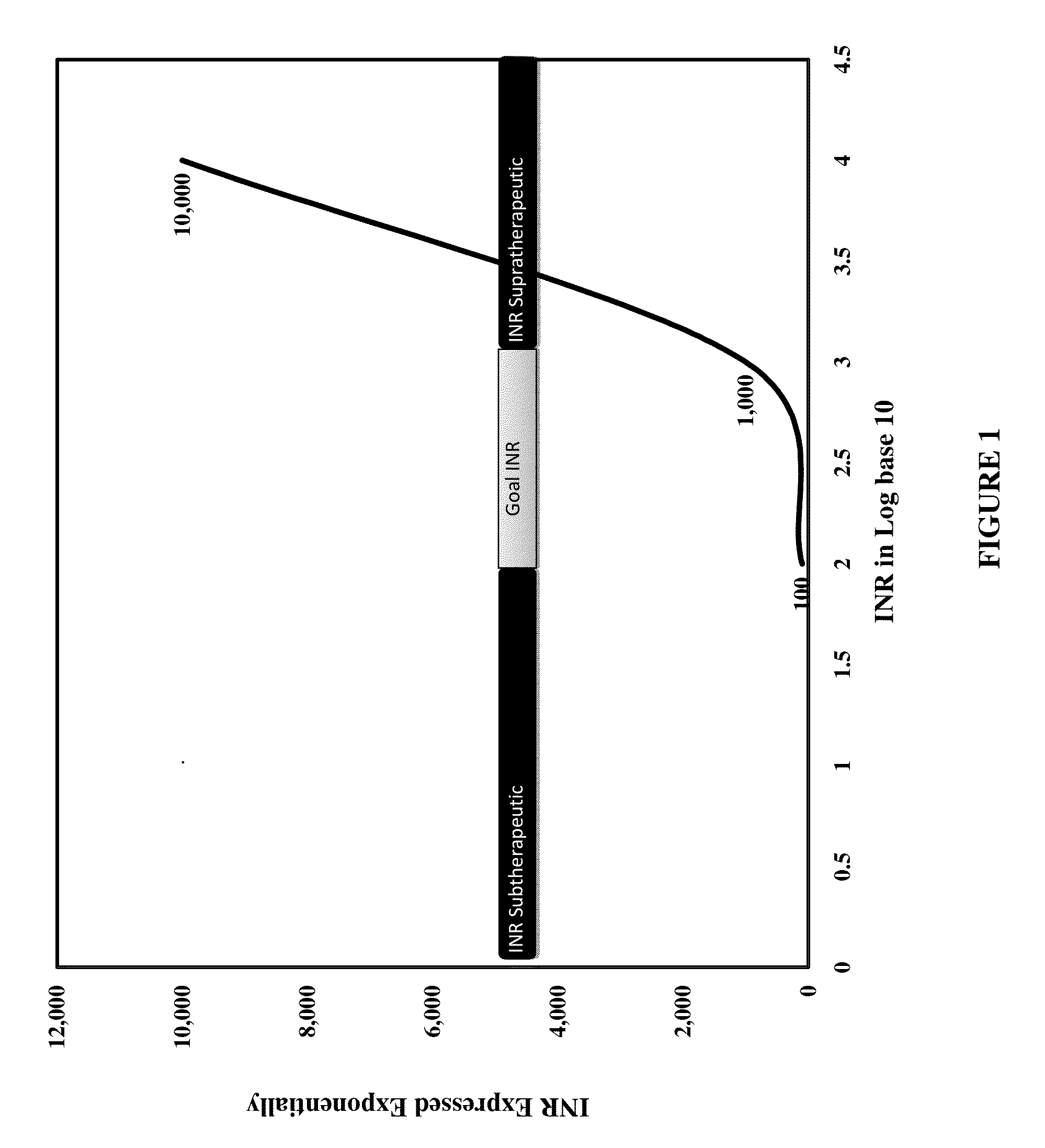
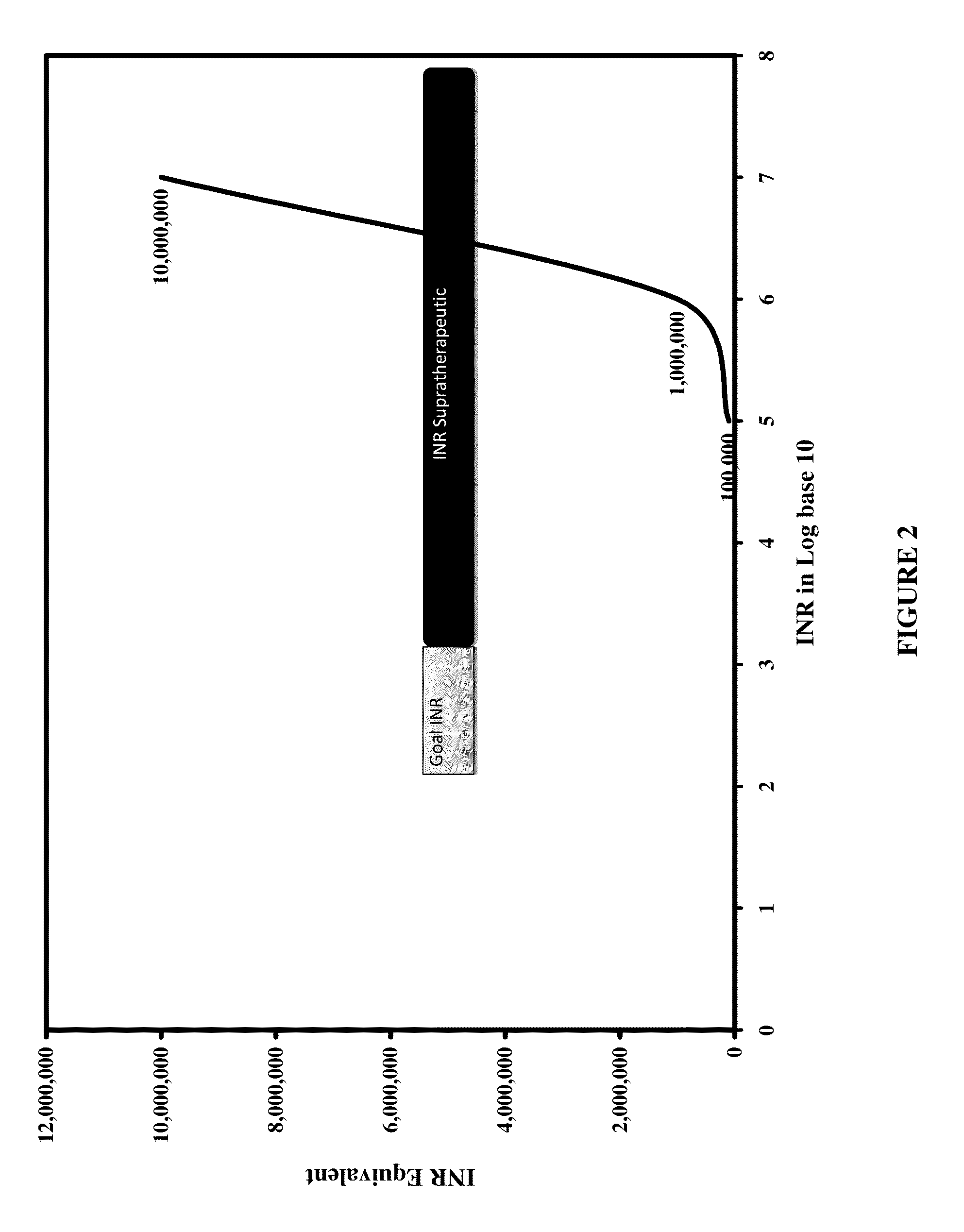
Individualized Dosing Technique With Multiple Variables
InactiveUS20130179184A1Data processing applicationsDrug and medicationsPersonalizationMultiple linear regression analysis
A method to quantify and correct for drug-drug interaction, physiologic change, diet, weight, genetic data and compliance on Warfarin dosing. The International Normalized Ratio (INR), a lab value used to follow Warfarin use, will fluctuate in an unpredictable manner due to factors other than the current Warfarin dose. The method mathematically describes these changes and eventually adjusts for these interacting factors through the use of logistic regression (LR) or multiple linear regression analysis. By anticipating changes in INR, Warfarin dosing can be adjusted resulting in patients having their INR be therapeutic range. The technique can be used in any field that requires a specific measured quantity, with variables that change and the need to correct for changes with a mathematical model.
Owner:HURST KATHERINE L
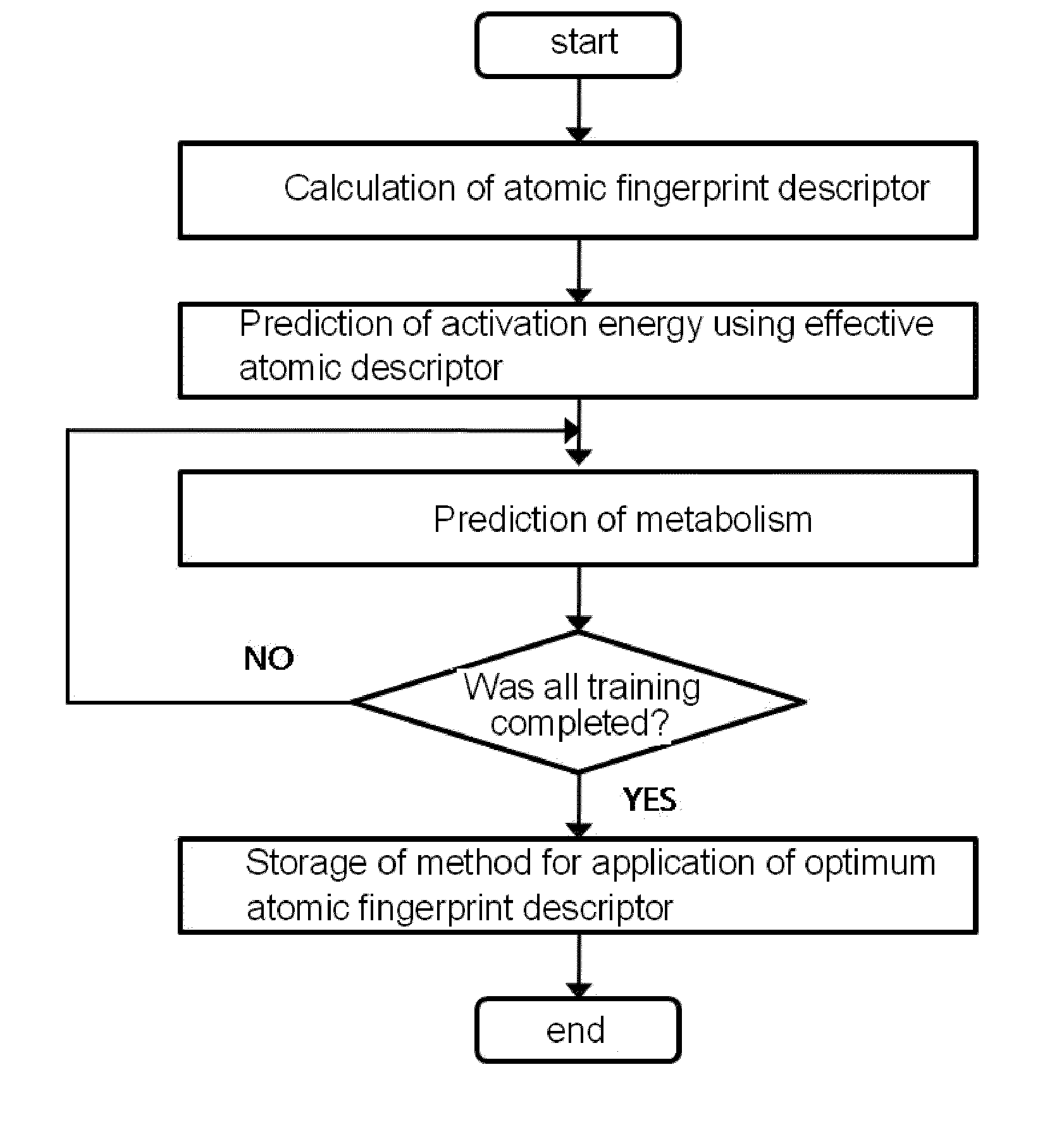
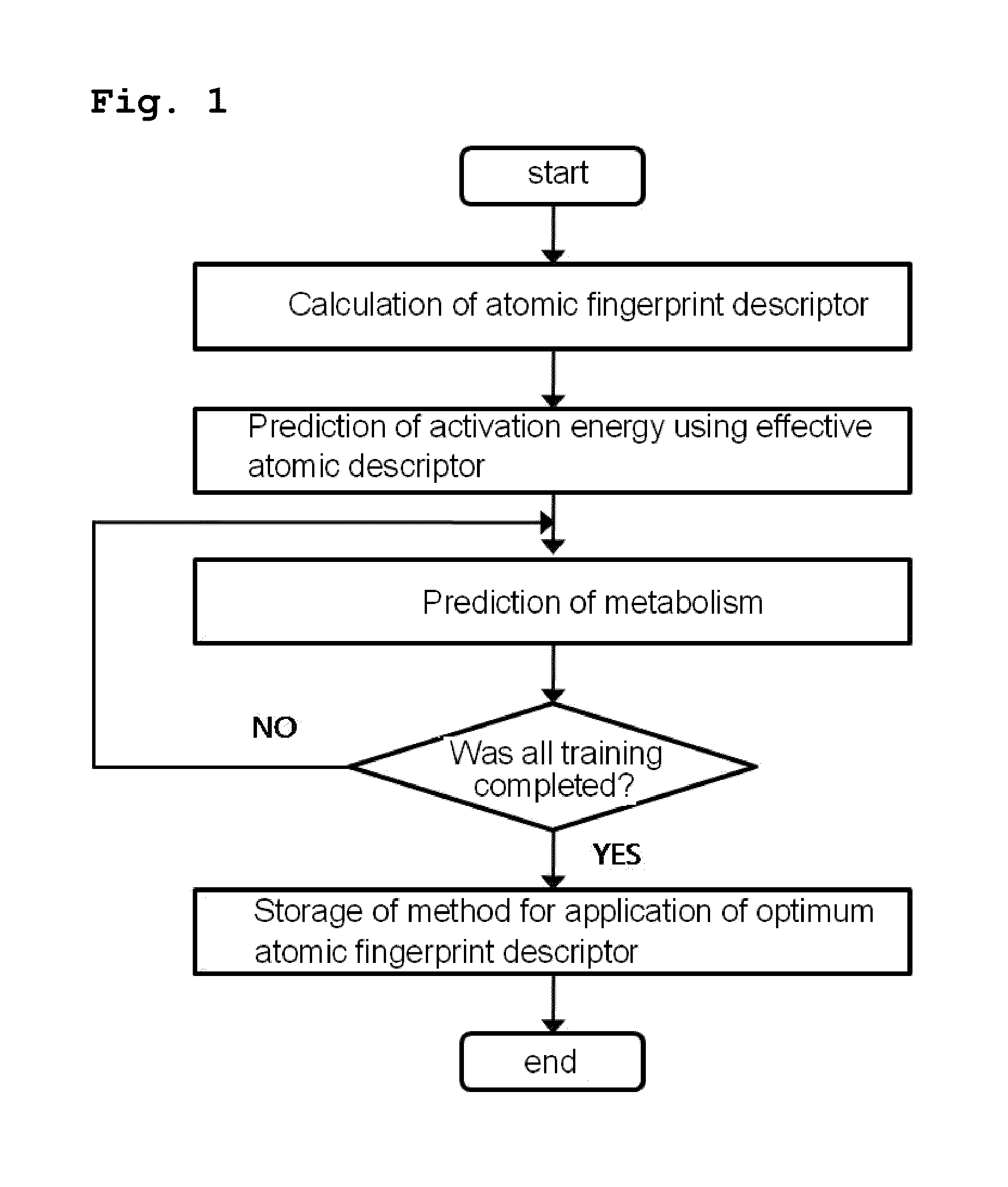
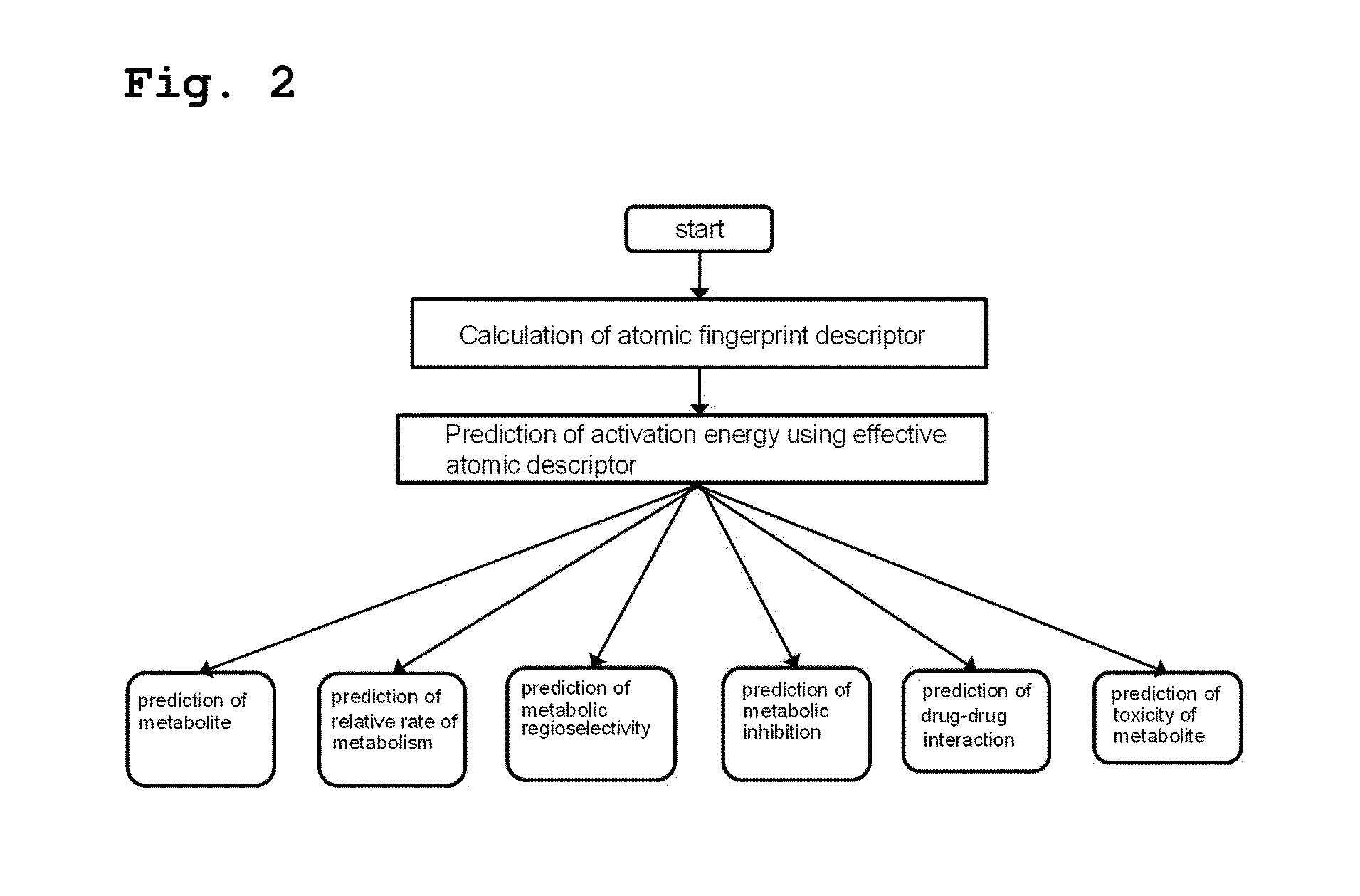
Method for predicting activation energy using an atomic fingerprint descriptor or an atomic descriptor
InactiveUS20110213558A1The process is fast and accurateChemical property predictionMolecular designMetaboliteChemical reaction
The present invention provides a method for constructing a database of atomic fingerprint descriptors. The invention provides a method for predicting activation energy using an atomic fingerprint descriptor and an atomic descriptor, the method comprising the steps of: (i) calculating the atomic fingerprint descriptor of a substrate; (ii) comparing the calculated atomic fingerprint descriptor with the constructed atomic fingerprint descriptor database to select an atomic position where cytochrome P450-mediated metabolism occurs; and (iii) predicting activation energy for the selected atomic position using an atomic descriptor. Also, the invention provides a method of predicting the activation energy of CYP450-mediated phase I metabolism using effective atomic descriptors. Specifically, the invention provides a method of predicting the activation energy either for cytochrome P450-mediated hydrogen abstraction or for tetrahedral intermediate formation in cytochrome P450-aromatic hydroxylation using equations including effective atomic descriptors. The method of the invention can rapidly predict activation energy for phase I metabolites at a practical level without having to perform a docking experiment between any additional CYP450 and the substrate, or a quantum mechanical calculation, thereby making it easier to develop new drugs using a computer. Also, the present invention may propose a strategy for increasing the bioavailability of drugs through the avoidance of metabolites based on the possibility of drug metabolism. Furthermore, the method of the present invention proposes new empirical approaches which can also be easily applied to activation energies for various chemical reactions, and makes it possible to explain physical and chemical factors that determine activation energy. In addition, through the prediction of activation energy according to the present invention, it is possible to predict i) metabolic products, ii) the relative rate of metabolism, iii) metabolic regioselectivity, iv) metabolic inhibition, v) drug-drug interactions, and vi) the toxicity of a metabolite.
Owner:BIOINFORMATICS & MOLECULAR DESIGN RES CENT
Physically dispersed, molecularly dissolved and/or chemically bound drug(s) in an empty, hard capsule shell composition
The present invention proposes a design to incorporate drug(s) in the hard capsule shells (body and cap) composition. Drug(s) in the cap and body of the capsule shell may be the same or may be different. Other drug(s) in the form of granules, beads etc. can be filled into the capsules as a core material. The drug(s) in the capsule core material may be the same as in the shell-composition or may be different. Thus, the same capsule may contain different drug(s) as the core material and in the shell. The key advantages of incorporation of drug in the capsule shell compositions are to minimize drug-drug interaction and to obtain a desired rate of release of the drug(s), mainly for potent ones. The concept can be applied to the hard gelatin, and hard non-gelatin capsules.
Owner:JOSHI HEMANT N
Method for treating a protozoal infection
InactiveUS20160354370A1Good for healthPatient benefitPeptide/protein ingredientsKetone active ingredientsSide effectKidney
Patients inflicted with various clustering chronic diseases require treatment with multiple drugs having distinct mechanisms of action. Accordingly, patients with multiple conditions suffer from cumulative side effects of multiple drugs as well as drug-drug interactions. Embodiments, agents, compounds or drugs of the present invention, such as sesquiterpenes, e.g., Zerumbone, replace an equal or larger number of approved drugs during patient treatment. Examples of disorders prevented or ameliorated by administration of the formulations of this invention include but are not limited to inflammatory diseases that may be, oncological, genetic, ischemic, infectious, neurological, hematological, ophthalmological, rheumatoid, orthopedic, neurological, hematological, kidney, vascular, dermatological, gynecological, or obstetric. The present invention further relates to a method of identifying agents, compounds or drugs useful in preventing or treating CDCP related diseases and conditions as well as other disorders, diseases and conditions treatable or preventable by the same agents, compounds or drugs.
Owner:REID CHRISTOPHER BRIAN
Cloud based emr manager
InactiveUS20160188844A1Well formedData processing applicationsComputer-assisted medical data acquisitionMedical recordDrug-drug interaction
Methods are directed at utilizing a patient's electronic medical record (EMR) information accessed from a cloud, such as that used in Blue Button technology. Once the patient's EMR information is downloaded from the cloud, it is used to automatically determine if the patient is eligible to participate in a clinical trial. The EMR information of the patient is also utilized to determine if there is a potential drug-drug interaction from of the one or more medications the patient is prescribed to take. Furthermore, the patient's EMR information is used to identify any warnings and indications regarding the one or more medications the patient is prescribed to take. Notifications are then sent to a patient device and devices of those that have permission from the patient to receive such information, such as family members.
Owner:CERNER INNOVATION
Screening system for identifying drug-drug interactions and methods of use thereof
InactiveUS20080194421A1Relieve painCompound screeningApoptosis detectionDrug interactionDrug-drug interaction
The invention features a method of screening for drug-drug interactions using combinational arrays. The method includes the steps of: (a) receiving a test drug from a client; (b) contacting the test drug and at least 200 library drugs from a drug library in an assay under conditions that ensure that each test drug / library drug contacting is segregated from the others; (c) recording the result of the contacting step (b); (d) identifying combinations of drugs that produce a result in the assay that is different from the results produced by either drug of the combination by itself, wherein each identified combination indicates an interaction between the test drug and the library drug of the combination; and (e) communicating the results of the identifying step (d) to the client.
Owner:BORISY ALEXIS +3
Method for Measuring Bile Salt Export Transport and/or Formation Activity
A method is provided to measure modulation of bile salt export transport and / or formation activity in hepatocyte or stable cell line preparations by test agents including but not limited to drugs, drug candidates, biologicals, food components, herb or plant components, proteins, peptides, DNA, RNA. Furthermore, the method is to determine modulation of bile salt export transport and / or formation activity not only by said test agents, but further their metabolites or biotransformed products formed in situ. The bile salt export transport and / or formation activity modulation includes but not limited to inhibition, induction, activation and / or regulation. The method can be practiced to identify test agents, which have potential to cause liver injury, drug-drug interactions, and / or can be used as therapeutic agents for the treatment of cholestasis, abnormality of bile salt metabolism, liver diseases and cholesterol abnormality.
Owner:BIOTRANEX
Drug interaction assay chip
InactiveUS20030059846A1Sequential/parallel process reactionsOrganic chemistry methodsDrug interactionFluorescence
A tool (a method, and a microarray apparatus) to enable evaluation of drug-drug interactions, by providing large numbers of pharmaceutical compounds on solid substrates in numerous replicate sets suitable for long-term storage. Ordinarily, the various compounds are present in extremely high densities. The libraries of pharmaceutical compounds, when used as a bioreaction assay chip, can be combined with a detection system that includes cells, cellular fractions, enzymes, organic molecules, and fluorescence or chromogenic reporter molecules along with a test drug agent. This method allows the detection of biochemical or biological interaction of the test drug agent with known pharmaceutical compounds in a defined biochemical or biological context of the assay.
Owner:MOREWOOD MOLECULAR +1
Prediction of potential drug-drug interactions using gene expression profiling of drug transporters, cytochrome p450s and nuclear x receptors
InactiveUS20100304984A1Sugar derivativesMicrobiological testing/measurementDrug-drug interactionCytochrome P450
The invention provides materials and methods for detecting the expression of genes encoding cytochrome p450, nuclear X receptors, phase H transferases, and solute carrier family uptake pumps. The materials include sets of primers, PCR amplicons and arrays. The methods of the invention include hybridization assays. Kits and assays for the detection of the expression of the genes are also provided by the invention. In addition, the invention provides the use of the materials and methods of the invention in drug screening assays.
Owner:NOAB BIODISCOVERIES
Tailored drug therapies and methods and systems for developing same
InactiveUS20160034667A1Reduce adverse side effectsData processing applicationsDrug and medicationsDrug-drug interactionPharmacogenomics
Several embodiments disclosed herein relate to methods of providing an optimized drug therapy that is specialized or customized for an individual subject or a group of subjects based, at least in part, on one or more of the genetic profile of the subject, the pharmacogenomic profile of the subject, and / or evaluation of possible drug-drug interactions. In several embodiments, systems specialized for performing one or more aspects of the methods are provided.
Owner:COMPANION DX REFERENCE LAB
Low dosage combinations of fluoxetine and reboxetine for treating obesity
ActiveUS20150335649A1Reduce adverse drug reactionsReduce interactionOrganic active ingredientsNorepinephrine reuptake inhibitorBULK ACTIVE INGREDIENT
The present invention provides a pharmaceutical composition comprising a selective serotonin reuptake inhibitor (SSRI) and a norepinephrine reuptake inhibitor (NRI), particularly, fluoxetine and reboxetine, for treating obesity. Surprisingly, the inventor of the present invention discovered that use of especially low doses of the active compounds, particularly, at most 6 mg / day of reboxetine and at most 20 mg / day of fluoxetine, wherein the reboxetinerfluoxetine ratio is from about 1:4 to about 1:6, induces an effective weight loss in obese patients. Advantageously, the combinations of the present invention include very low doses of the active ingredients, thereby decreasing possible drug-drug interactions and adverse drug reaction.
Owner:BARAK NIR
Method for measuring bile salt export transport and/or formation activity
A method is provided to measure modulation of bile salt export transport and / or formation activity in hepatocyte or stable cell line preparations by test agents including but not limited to drugs, drug candidates, biologicals, food components, herb or plant components, proteins, peptides, DNA, RNA. Furthermore, the method is to determine modulation of bile salt export transport and / or formation activity not only by said test agents, but further their metabolites or bio transformed products formed in situ. The bile salt export transport and / or formation activity modulation includes but not limited to inhibition, induction, activation and / or regulation. The method can be practiced to identify test agents, which have potential to cause liver injury, drug-drug interactions, and / or can be used as therapeutic agents for the treatment of cholestasis, abnormality of bile salt metabolism, liver diseases and cholesterol abnormality.
Owner:BIOTRANEX
Stable pharmaceutical composition for atherosclerosis
The present invention relates to a stable solid oral pharmaceutical multi-component composition comprising combination of blood pressure lowering drugs with lipid lowering agent / s and optionally a platelet aggregation inhibitor in a single dosage form. The blood pressure lowering agents are selected from β-adrenergic receptor blocking agent, ACE inhibitor and diuretic. The lipid lowering agent is selected from HMG Co-enzyme-A reductase inhibitor. The pharmaceutical composition made as per present invention a) overcomes any drug-drug interactions, b) exhibits pharmacokinetic and pharmacodynamic profile of individual therapeutic agent, c) has minimal side effects. The invention provides multi-component composition (MCC) to increase adherences to therapy. The MCC as per present invention provides compositions that maintain activity of all active ingredients without significant increase in adverse event profile. The present invention further relates to a method of preparing the said pharmaceutical composition.
Owner:CADILA PHARMA
Construction and application of cell model capable of stably expressing human MATE1 transporter
InactiveCN104212834AMicrobiological testing/measurementVector-based foreign material introductionDrug-drug interactionResearch model
The invention provides a method for constructing a pcDNA3.1(+)-hMATE1 recombinant plasmid. The pcDNA3.1(+)-hMATE1 recombinant plasmid is used for constructing three cell models: MDCK-hMATE1, MDCK-hOCT1 / hMATE1 and MDCK-hOCT2 / hMATE1. Stably high expression of hMATE1 on MDCK cells can be carried out with the constructed MDCK-hMATE1 model. The MDCK-hMATE1 model can be used as a research model for screening a substrate and an inhibitor of the hMATE1 at a high speed and a high flux, can predict a drug-drug interaction induced by the hMATE1. A double-transfection cell model of the MDCK-hOCT1 / hMATE1 and the MDCK-hOCT2 / hMATE1 in the invention can be used for researching transfer of a drug of common substrates of the hOCT1 / hOCT2 and the hMATE1.
Owner:ZHEJIANG UNIV
Method for acquiring and analyzing a list of a patient's prescription medications
A computer system for obtaining, analyzing and providing information to a community of user patients regarding their medication is disclosed. The system is provided by means of world wide web access and generates a user patient screen prompting the manual entry of data relating to the use patient and drugs being taken. The data are analyzed and results are provided to the user patient and / or the caregiver including drug-drug interactions, drug pricing, alternative medications and possible adjustments in the dosing regimen of the user patient.
Owner:RUBSAMEN REID M
Organic anion transporter and gene coding for the same
InactiveUS20060057677A1Cell receptors/surface-antigens/surface-determinantsSugar derivativesDrug-drug interactionIn vitro analysis
A protein capable of transporting organic anions having amino acid sequences represented by SEQ ID NO: 1 or 2 or amino acid sequences derived therefrom by deletion, substitution or addition of one or more amino acid residues; and a gene coding for the protein. The protein and gene therefor are useful in vitro analysis of drug release and drug-drug interactions and development of methods for screening drugs useful for preventing nephrotoxicity.
Owner:FUJI BIOMEDIX
RAT GENE EXPRESSION PROFILING OF DRUG TRANSPORTERS, CYTOCHROME P450s, TRANSFERASES AND NUCLEAR XENOBIOTIC RECEPTORS FOR PREDICTING DRUG EFFECTS
InactiveUS20100160176A1Predict potentialTo promote metabolismNucleotide librariesMicrobiological testing/measurementPrimary cellCytochrome p450 enzyme
The disclosure describes materials and methods for detecting the expression of genes and generating a gene expression profile from drug-treated rat primary cells or established rat cell lines using a unique combination of rat cytochrome p450 enzyme, nuclear xenobiotic receptor, transferase and transporter gene sequences. The materials include sets of primers, PCR amplicons and arrays. The methods include hybridization assays. Assays for the detection of the expression of the genes are also provided. In addition, the disclosure provides the use of the materials and methods in drug screening assays and, specifically, the detection of potential drug-drug interaction(s).
Owner:NOAB BIODISCOVERIES
A renal cell line with stable transporter expression
InactiveUS20180362935A1Improve variationImprove prediction of nephrotoxic potentialCell receptors/surface-antigens/surface-determinantsGenetically modified cellsDrug interactionDrug-drug interaction
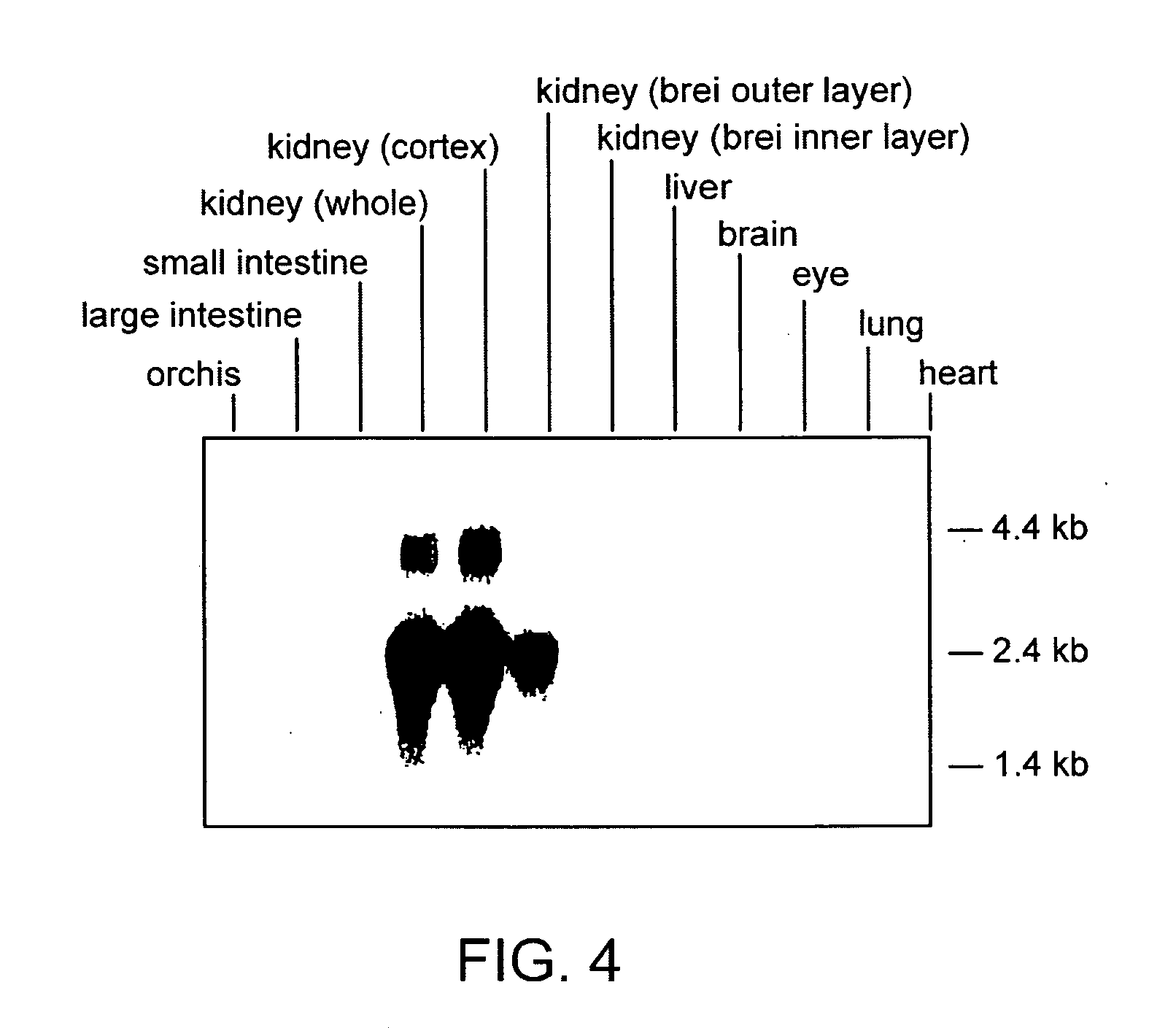
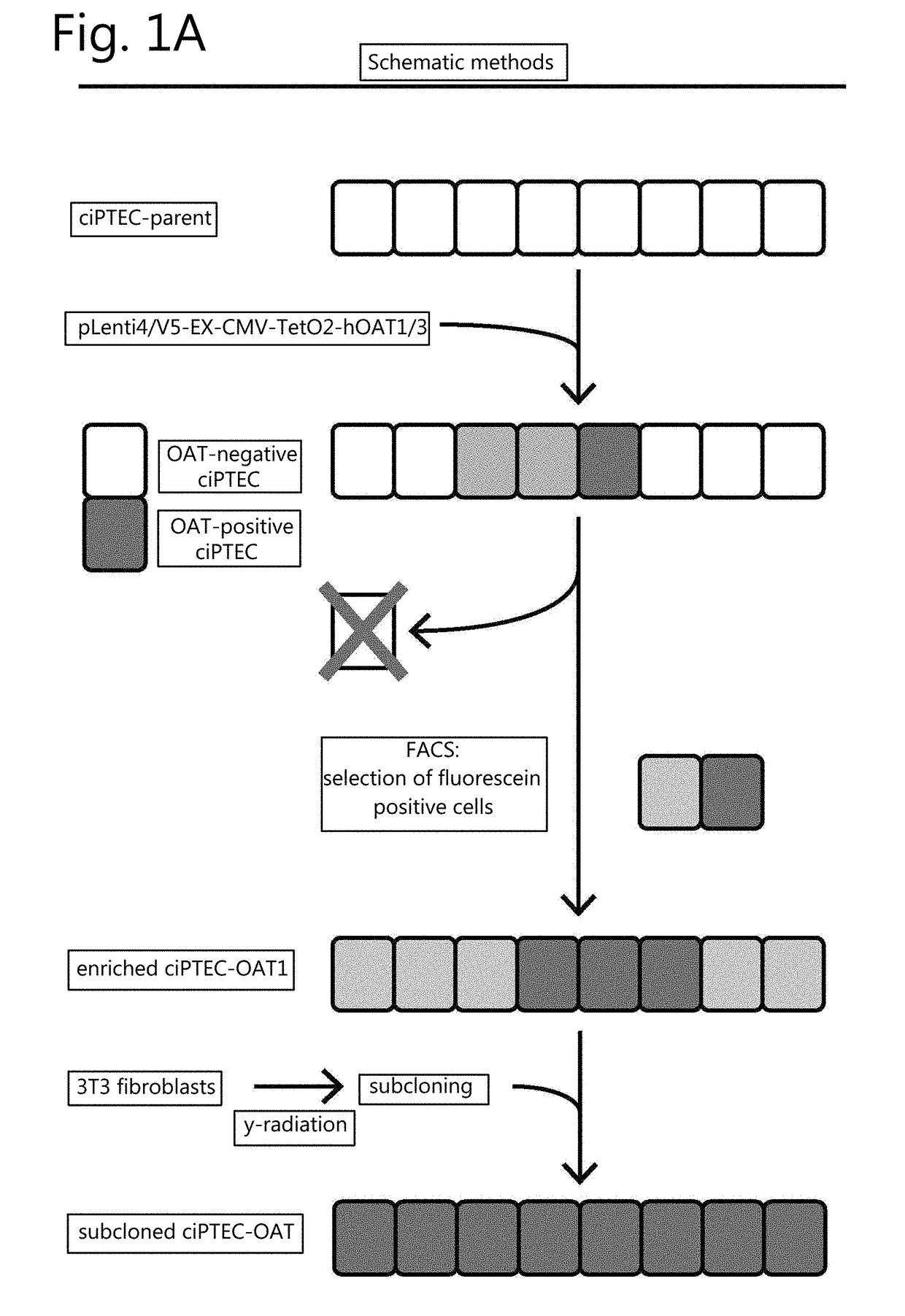
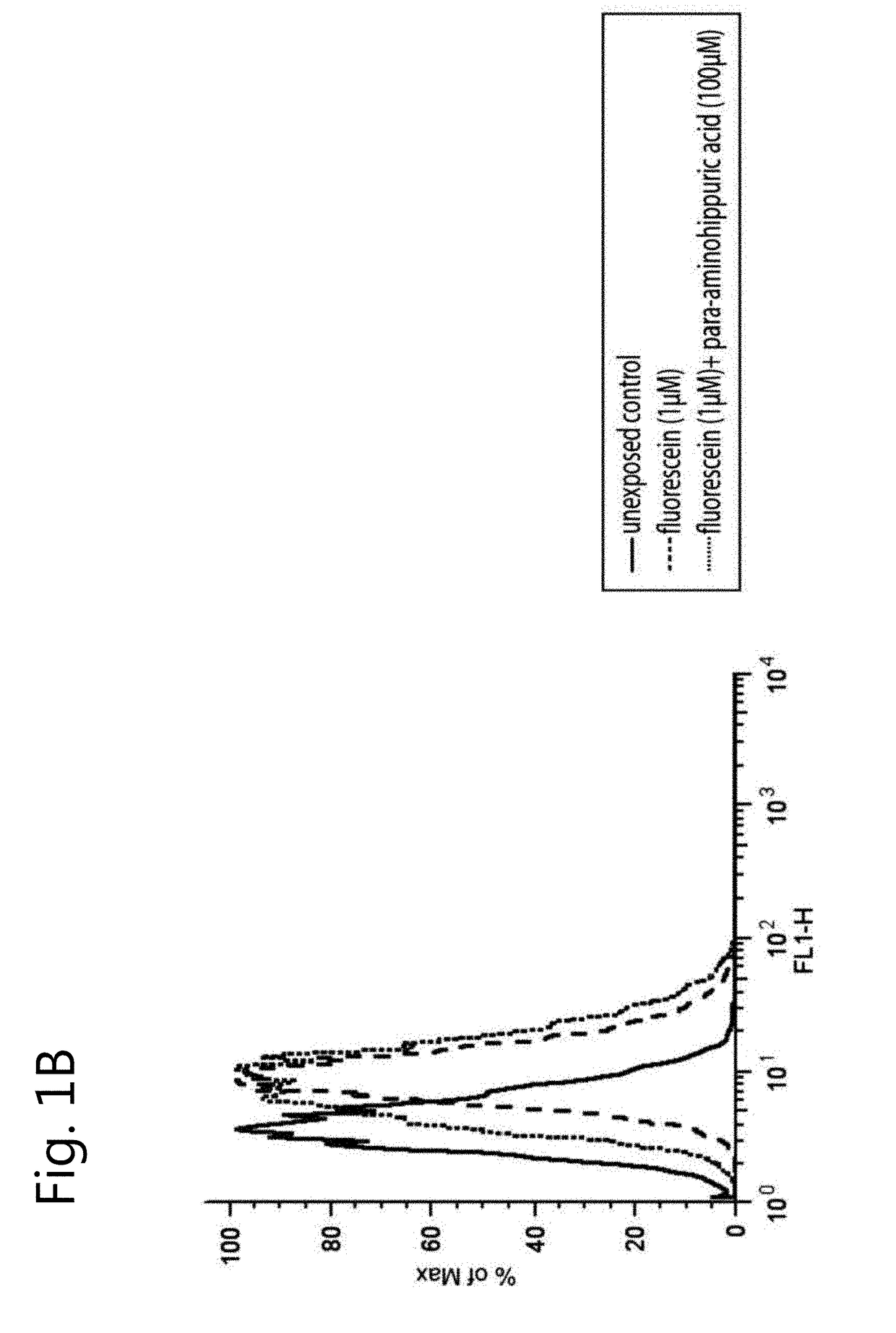
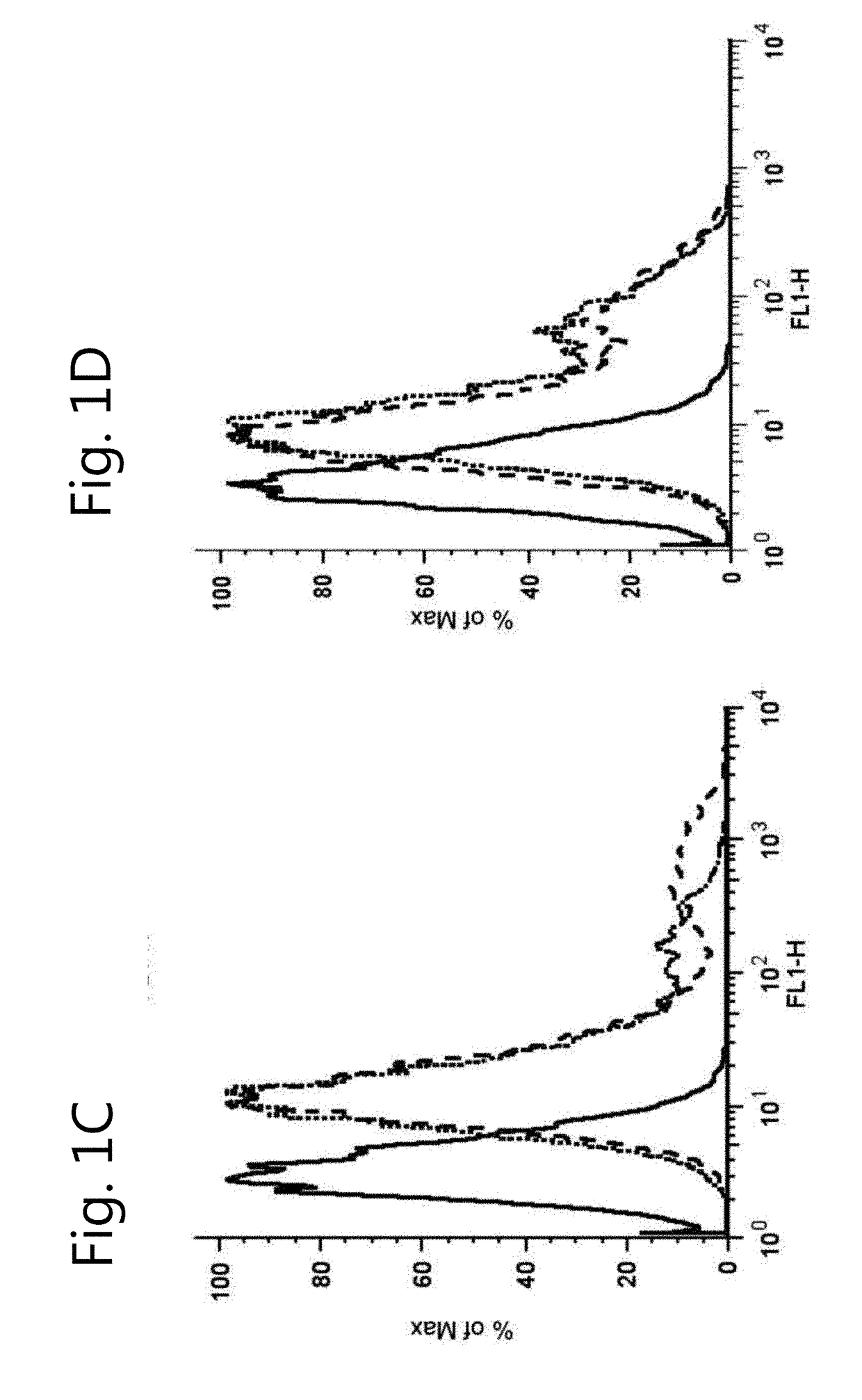
The invention relates to the field of pharmacology, specifically the field of drug-drug interactions and nephrotoxicity. An engineered, stable cell line of human renal cells is provided that allows screening for drug-drug interactions and nephrotoxicity.
Owner:STICHTING KATHOLIEKE UNIV
Ibuprofen and phenylephrine hydrochloride-containing double-layer medicinal composition and preparation method thereof
InactiveCN106943388ARelieve nasal congestionReduce interactionOrganic active ingredientsAntipyreticDrug-drug interactionFast release
The invention provides an ibuprofen and phenylephrine hydrochloride-containing double-layer medicinal composition. The ibuprofen and phenylephrine hydrochloride-containing double-layer medicinal composition is characterized in that the ibuprofen and phenylephrine hydrochloride-containing double-layer medicinal composition is a double-layer tablet consisting of an ibuprofen sustained-release layer and a phenylephrine hydrochloride fast-release layer; the ibuprofen sustained-release layer contains 100mg-500mg of ibuprofen; and the phenylephrine hydrochloride fast-release layer contains 5mg-30gm of phenylephrine hydrochloride. The ibuprofen and phenylephrine hydrochloride-containing double-layer tablet has the beneficial effects that the ibuprofen and the phenylephrine hydrochloride are respectively prepared into particles or powder, and then the particles or powder is compressed into the double-layer tablet; the phenylephrine hydrochloride fast-release layer realizes the fast release of medicines, quickly takes effect and relieves symptoms such as nasal obstruction, running nose and sneezing; the ibuprofen sustained-release layer realizes the sustained release of medicines and persistently relieves cold associated symptoms such as headache, fever, nasal obstruction and mild body pain; and meanwhile, the double-layer tablet can reduce the drug-drug interaction and improves the stability of preparations.
Owner:南京康川济医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com