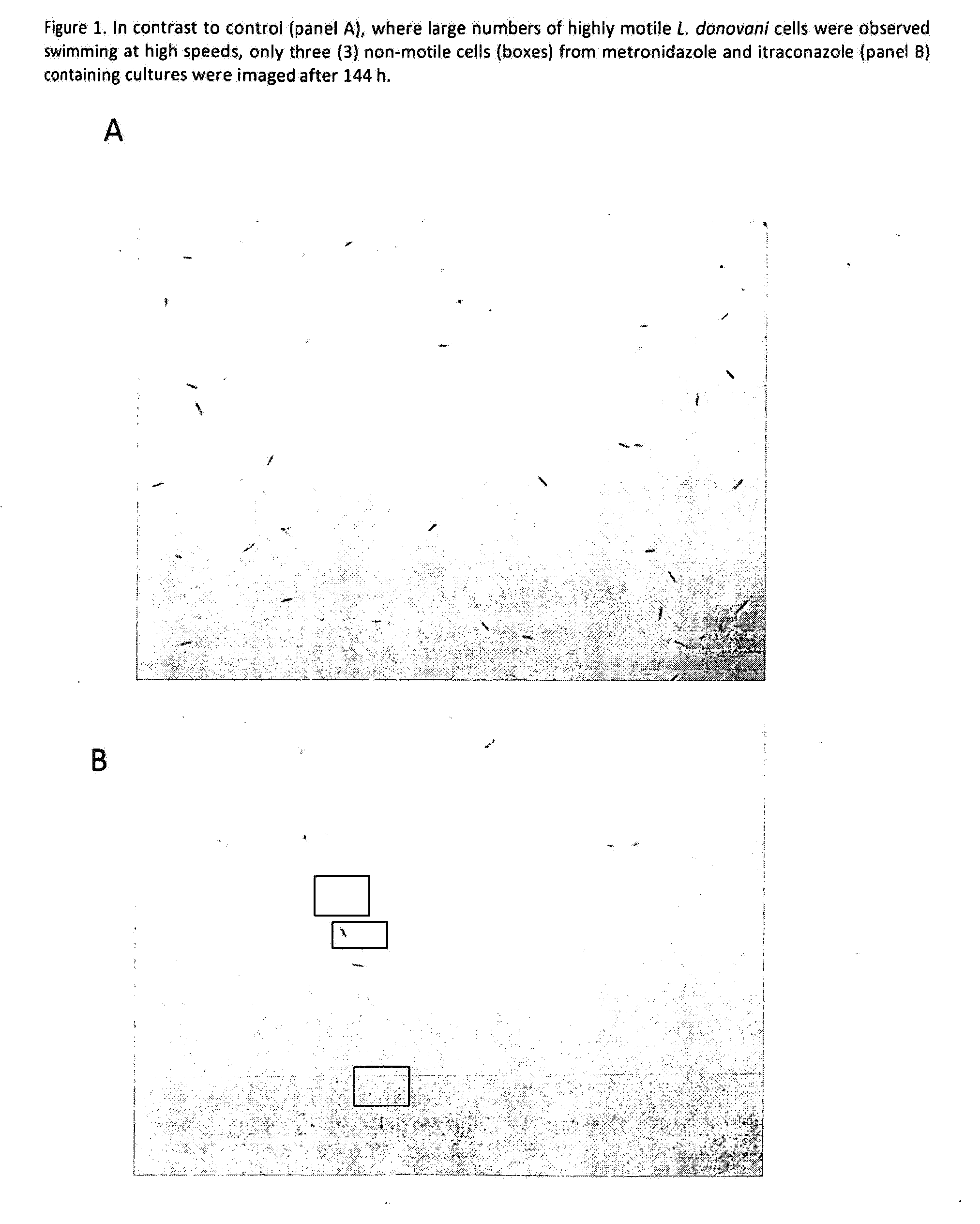
Method for treating a protozoal infection
a protozoal infection and protozoal technology, applied in the field of protozoal infection treatment, can solve the problems of increasing polypharmacy, increasing the risk factor for important morbidity and mortality, and virtually all medications have the potential to cause significant side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Solid Lipid Nanoparticles
[0338]In one embodiment, the method chosen for the preparation of nanoparticles is an adaptation of the w / o / w double emulsion technique (Garcia-Fuentes et al 2003; Zhang et al 2006; Sarmento et. al., 2007). Approximately 200 mg of acetyl palmitate is dissolved in about 4 mL of dichloromethane 7 mg of zerumbone, a sesquiterpene and / or an equivalent effective amount of other agent(s), compound(s), or drug(s) of the present invention and glutathione) are dissolved in 0.5 mL of HCL 0.1 M. The drug solution is added to the lipid solution and then homogenized for 30 seconds in an ultra-turrax T25 (IKA-Labortechnik, Germany) or a similar apparatus. The primary emulsion is then poured into 25 mL of 2% poloxamer 407 solution and homogenized for another 30 seconds. The solvent is subsequently discarded and the emulsion is concentrated in a rotavapor until ˜10 ml_. Optionally, particle size can be analyzed using photon correlation spectroscopy (PCS); and...
example 2
Preparation of a Liposomal Formulation
[0340]A liposomal formulation comprising the agent(s), compound(s), and drug(s) of the present invention may be prepared according to Good Manufacturing Practices by the method of (Paul et al. (1997), previously described by Fessi at al. (1988). Briefly, an organic phase containing phospholipids and the drugs is introduced under magnetic stirring in an aqueous phase. The organic solvent is evaporated, and the liposomes obtained are filtered and lyophilized). Prior to administration, 50 mg of lyophilized liposomes are resuspended in sterile distilled water (20 ml), shaken for 3 min, and then diluted in 5% dextrose.
example 3
Preparation of a Tablet Formulation
[0341]Compressed tablets containing the pharmaceutical composition of the invention may be prepared by uniformly mixing the active ingredient(s) with a solid carrier to provide a mixture. The mixture is then compacted to the shape and size desired. Molded tablets maybe made in a suitable machine. To prepare a tablet formulation containing agents, compounds, or drugs of the present invention, the selected active components (e.g. Zerumbone and / or other agent(s), compound(s), or drug(s) of the present invention (80 g) and reduced glutathione (400 g)) may be mixed in the dry state for 10 minutes in a blade mixer. Likewise, a solution is prepared containing gelatin (16 g), dioctyl sodium sulphosuccinate (1 g), alcohol (57 g) and purified water (80 g). The solution is then wet mixed with the powders for 10 minutes using a slow speed. The wet mass is passed through a 1000 urn screen. Subsequently, the granules are dried in a fluidized bed at 60° C. for 30...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| conductivity | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com