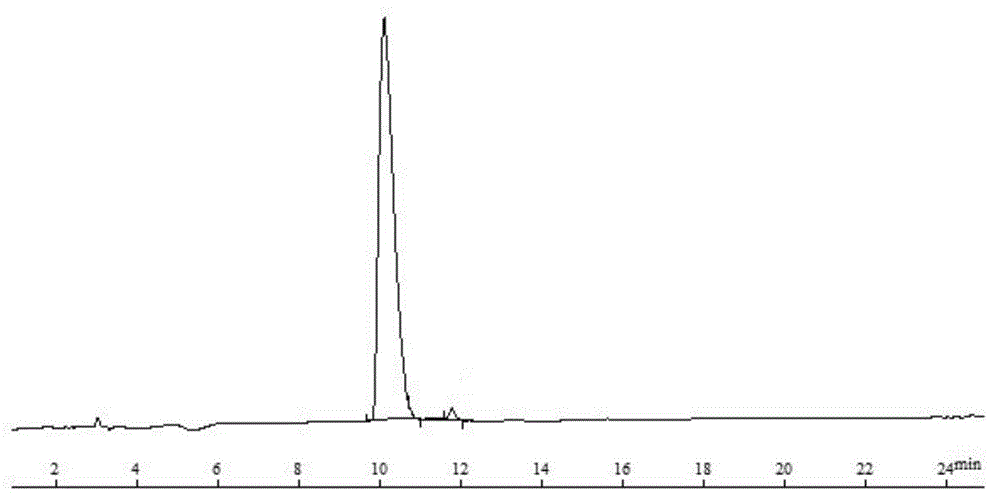
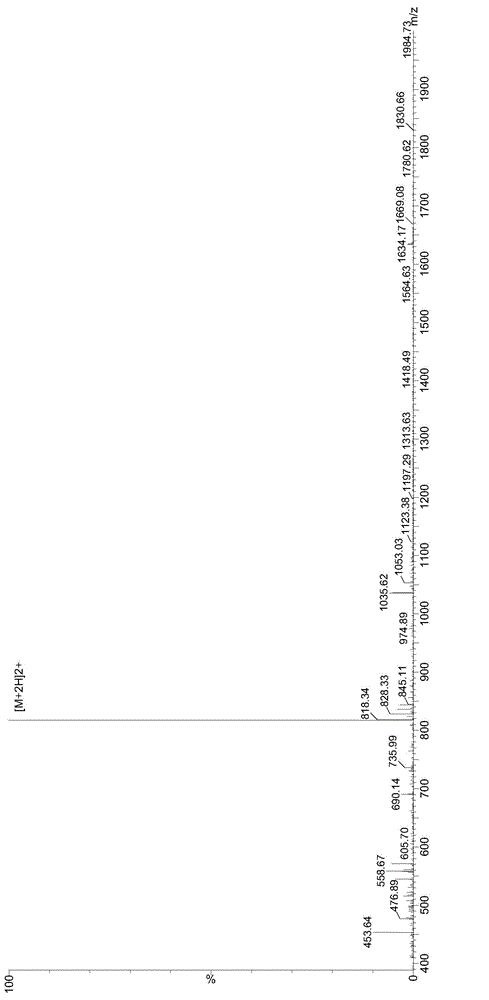
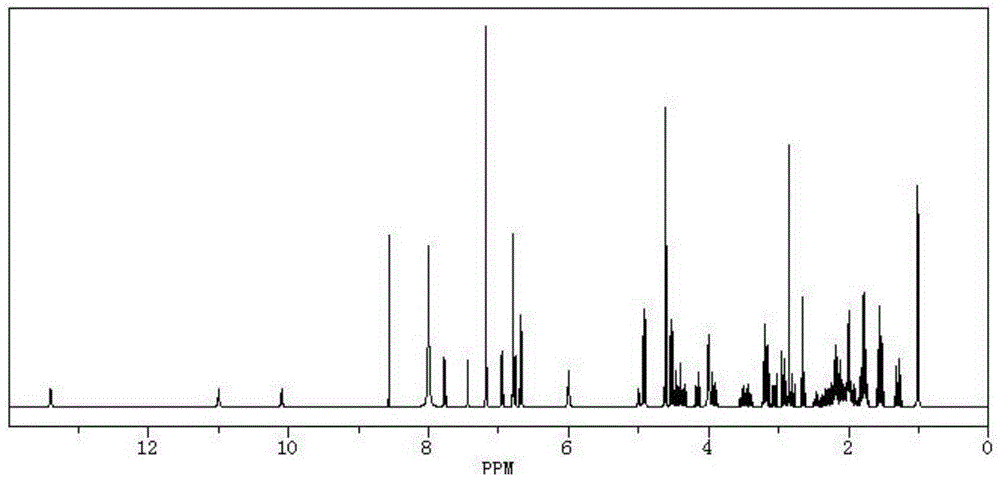
GnRH polypeptide-methotrexate conjugate, and preparation method and application thereof
A technology of methotrexate and conjugates, applied in the field of GnRH polypeptide-methotrexate conjugates, which can solve the problems of normal tissue and organ toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation of embodiment 1 methotrexate active ester
[0063] Example 1 Set three groups in parallel (Examples 1.1, 1.2, 1.3), 454 mg (1 mmol) of methotrexate was dissolved in 20 mL of DMSO, and 575.1 mg (3 mmol) of EDC.HCl reagent was added, respectively at 25 ° C, 35 ° C and React at 45°C for two hours, add 345.27mg (3mmol) NHS and 61.22mg (0.5mmol) DMAP after two hours, the whole reaction is carried out under anhydrous, oxygen-free and light-shielding conditions, follow the reaction with thin layer chromatography (TLC) , stop reaction after reacting 24h, suction filtration, remove unreacted solid reagent, then dilute reaction solution with diethyl ether acetone mixed solvent, anhydrous MgSO Dry 24h, suction filtration, rotary evaporation removes solvent, silica gel column chromatography (V ethyl acetate: V petroleum ether=1:6) separation and purification to obtain a yellow liquid, weigh the weight of methotrexate active ester, calculate the reaction yield, the r...
Embodiment 2
[0067] The preparation of embodiment 2 methotrexate active esters
[0068] Example 2 Set up three groups in parallel (Example 2.1, 2.2, 2.3), methotrexate 423.66mg (0.93mmol) was dissolved in 20mLDMSO, added the EDC.HCl reagent of 534.8mg (2.79mmol), and the reaction time at room temperature 2 hours respectively, 321.12mg (2.79mmol) NHS and 57.42mg (0.47mmol) DMAP were added after 2 hours, the whole reaction was carried out under anhydrous, anaerobic and light-proof conditions, and the reaction was followed by TLC, followed by Example 2.1, 2.2 and 2.3 The reaction time of the three groups of reactions is set to 4 hours respectively. After 12 hours and 24 hours, the reaction is terminated, the unreacted solid reagent is removed by filtration, and then the reaction solution is diluted with a mixed solvent of ether and acetone, and dried with anhydrous MgSO4 for 24 hours. Suction filtration, rotary evaporation to remove solvent, silica gel column chromatography (V ethyl acetate:V...
Embodiment 3
[0072] The preparation of embodiment 3 methotrexate active esters
[0073] Example 3 Set three groups in parallel (Example 3.1, 3.2, 3.3), methotrexate 448.6mg (0.988mmol) was dissolved in 20mLDMSO (containing 0.2mL of triethylamine), added 465mg (2.96mmol) of EDC. HCl reagent, reaction at room temperature for two hours, add 345.27mg (3mmol) NHS after two hours, the amount of adding DMAP respectively in embodiment 3.1, 3.2 and 3.3 is 61.1mg (0.5mmol), 122.2mg (1.0mmol) and 183.3mg ( 1.5mmol), the whole reaction is carried out under anhydrous, anaerobic and light-shielding conditions, follow the reaction with TLC, terminate the reaction after 24h of reaction, remove unreacted solid reagent by filtration, then dilute the reaction solution with diethyl ether acetone mixed solvent, no Water MgSO Dry 24h, suction filtration, rotary evaporation to remove solvent, silica gel column chromatography (V ethyl acetate:V petroleum ether=1:6) separation and purification, obtain yellow liqui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com