Nanocrystalline of hydrophobic drug, as well as preparation and application methods of nanocrystalline
A technology of hydrophobic drugs and nanocrystals, which is applied in the field of insoluble hydrophobic drug nanocrystals and insoluble hydrophobic drug nanocrystals, can solve the problems of decomposition loss of effective components of drugs, drug pollution, and hydrophobic and insoluble drugs, and achieves good general applicability. high drug loading, narrow particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A paclitaxel nanocrystal preparation, prepared by the following method:
[0061] (1) Dissolve 20 mg of paclitaxel in 100 μL of dichloromethane, and mix with 10 mL containing 0.1wt% poloxamer The aqueous solution of F127 was mixed; the emulsification treatment was carried out by ultrasound with a power of 20W to obtain an O / W type emulsion with paclitaxel dissolved in the oil phase;
[0062] (2) Soak the O / W type emulsion obtained in step (1) in liquid nitrogen to solidify, then immediately transfer to a freeze dryer to dry for 48 hours, remove the solvent molecules therein, and obtain the crude product of paclitaxel nanocrystals, and then process the crude product repeatedly Resuspension-centrifugation-resuspension for purification to obtain paclitaxel nanocrystal products, denoted as NPs;
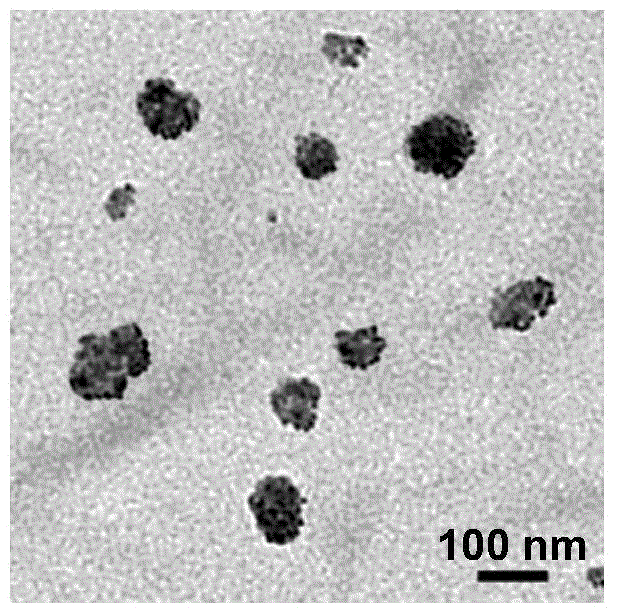
[0063] figure 1 It is a transmission electron microscope (TEM) photo of paclitaxel nanocrystals (NPs) in Example 1; the prepared paclitaxel nanocrystals have an average particle ...
Embodiment 2
[0065] A nanocrystal of iRGD functionalized paclitaxel, the difference between its preparation method and Example 1 is only that the loxamer Full or partial replacement of F127 with iRGD-modified poloxamer F127, the obtained iRGD-functionalized paclitaxel nanocrystals are denoted as iNPs.
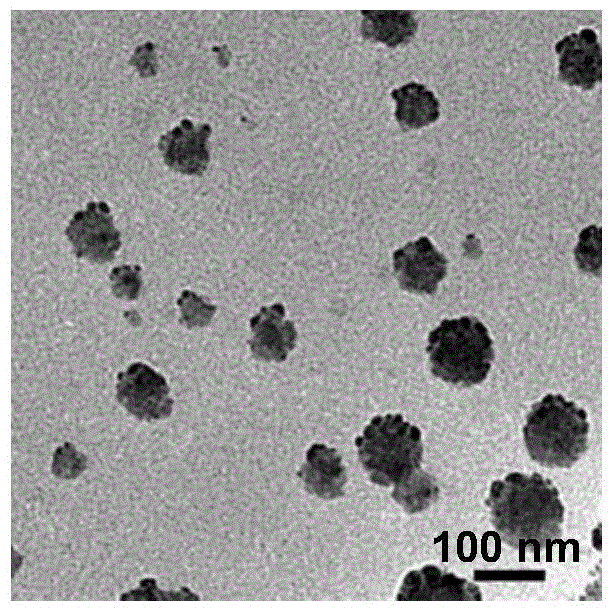
[0066] figure 2 It is a TEM photo of iRGD functionalized paclitaxel nanocrystals (iNPs) in Example 2; the average particle size of the prepared iRGD functionalized paclitaxel nanocrystals is 71.3nm, and the drug loading reaches 94.33wt%.
Embodiment 3
[0068] A paclitaxel nanocrystal preparation, prepared by the following method:
[0069] (1) Dissolve 1mg of paclitaxel in 1mL of dichloromethane, and mix with 1mL containing 2.0wt% poloxamer The aqueous solution of F127 was mixed and emulsified by ultrasonic power of 100W to obtain an O / W emulsion with paclitaxel dissolved in the oil phase;
[0070] (2) Soak the O / W type emulsion obtained in step (1) in liquid nitrogen to solidify, then immediately transfer to a freeze dryer to dry for 48 hours, remove the solvent molecules therein, and obtain the crude product of paclitaxel nanocrystals, and then process the crude product repeatedly Resuspension-centrifugation-resuspension for purification to obtain paclitaxel nanocrystal products, denoted as NDs;
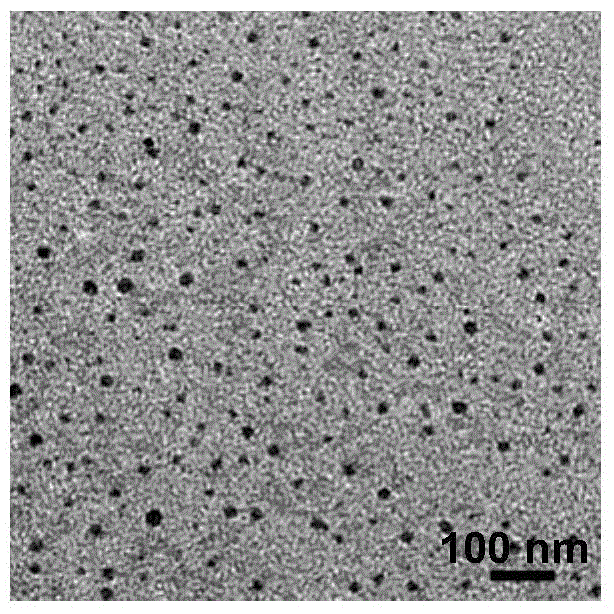
[0071] image 3 It is a TEM photo of paclitaxel nanocrystals (NDs) in Example 3; the average particle size of the prepared paclitaxel nanocrystals is 8.6nm, and the drug loading capacity reaches 80.57wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com