Synthesis of D(-)-alpha-(4-ethyl-2,3-dioxygen ethylene imine-1-formamido) p-hydroxybenzene acetic acid
A technology of p-hydroxyphenylacetic acid and p-hydroxyphenylglycine, which is applied in the synthesis field of D-α-p-hydroxyphenylacetic acid, can solve the problem of increasing reaction equipment and processing steps, increasing reaction raw materials and reaction steps, and increasing the ease of trimethylchlorosilane. Moisture absorption and decomposition and other problems, to achieve the effect of simplifying the production process, reducing consumption, and being environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 150g of p-hydroxyphenylglycine and 700mL of water into a 1000mL reaction flask, and adjust the pH value to 7.5 with 10% sodium hydroxide solution to clarify the solution. Cool the solution to 5°C, add 187.5g of 4-ethyl-(2,3-dioxopiperazinyl)formyl chloride-1 in batches, and maintain the pH of the system at 7.5 with solid sodium carbonate during the addition Between ~8, it takes about 2h to finish adding. After adding the materials, the reaction was continued at 5° C. for 2 h. After the reaction, add activated carbon for decolorization, and then adjust the pH of the reaction solution to 2 with 6mol / L hydrochloric acid solution, and white crystals are precipitated, filtered and dried to obtain D(-)-α-(4-ethyl-2,3- Dioxypiperazine-1-carboxamido)p-hydroxyphenylacetic acid (HO-EPCP) pure product 274.2g, yield 91%.
[0019] The content of HO-EPCP determined by HPLC method was 99.89%.
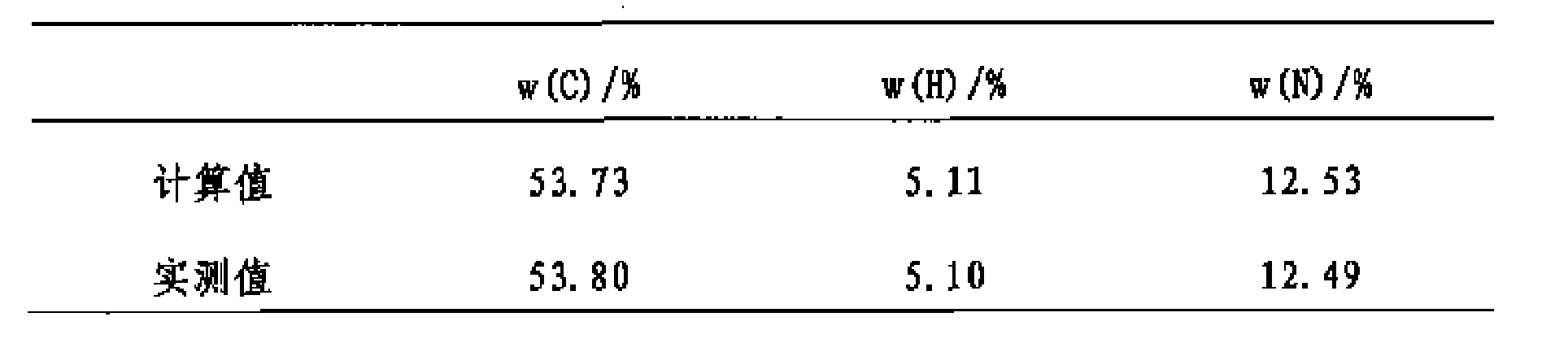
[0020] The characterization of the HO-EPCP product obtained by the reaction:
[002...
Embodiment 2
[0028] Add 150g of p-hydroxyphenylglycine and 650mL of water into a 1000mL reaction flask, and adjust the pH value to 7.5-8 with 10% potassium hydroxide solution to clarify the solution. Cool the solution to 2°C, add 190.21g of 4-ethyl-(2,3-dioxopiperazinyl)formyl chloride-1 in batches, and maintain the pH of the system at 7.5 with solid sodium carbonate during the addition Between ~8, it takes about 2h to finish adding. After adding the materials, the reaction was continued at 2 °C for 5 h. After the reaction, add activated carbon for decolorization, and then use 6mol / L hydrochloric acid solution to adjust the pH of the reaction solution to 1.5. White crystals are precipitated, filtered and dried to obtain 275.7g of pure HO-EPCP with a yield of 91.5%.
[0029] The detection content is 99.88%, the specific rotation .
Embodiment 3
[0031] Add 150g of p-hydroxyphenylglycine and 800mL of water into a 1000mL reaction flask, and adjust the pH value to 8-9 with 10% sodium hydroxide solution to clarify the solution. Cool the solution to 0-2°C, add 190.21g of 4-ethyl-(2,3-dioxopiperazinyl)formyl chloride-1 in batches, and maintain the pH of the system with solid sodium carbonate during the addition Between 7.5 and 8, it takes about 2 hours to finish adding. After adding the material, keep it at 0-2°C and continue the reaction for 3h. After the reaction, add activated carbon for decolorization, and then use 6mol / L hydrochloric acid solution to adjust the pH of the reaction solution to 1.9. White crystals are precipitated, filtered and dried to obtain 271.2 g of pure HO-EPCP with a yield of 90%.
[0032] The detection content is 99.95%, the specific rotation .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com