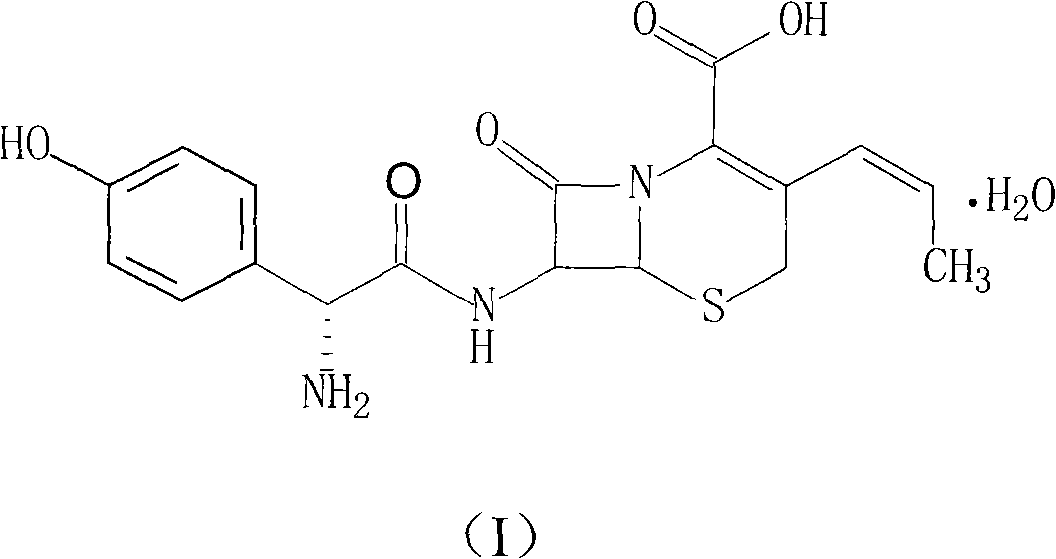
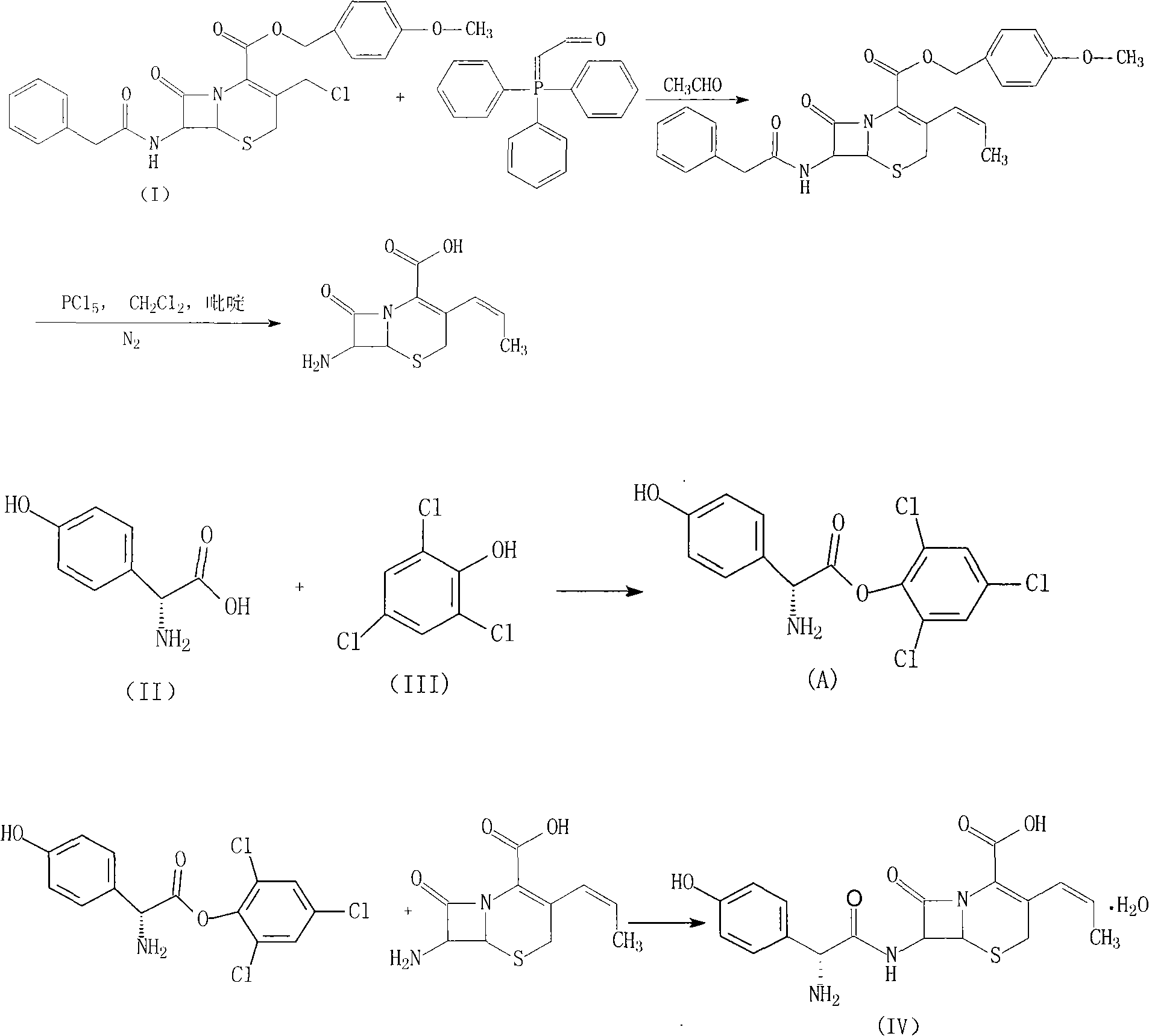
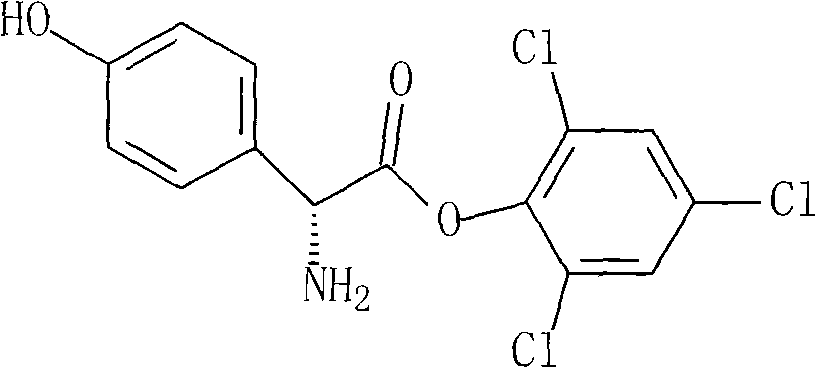
Novel route cefprozil compound
A technology of cefprozil and compounds, applied in the field of medicine, can solve the problems of large isomer content, only yield, and low product purity, and achieve the effects of small isomer content, easy scale-up production, and improved reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0037] 1. Preparation of Cefprozil
[0038] (1) Mix 50 g of p-methoxybenzyl 7-phenylacetamide-3-chloromethyl cephalosporin acid and 28.3 g of triphenyl phosphate, add 300 ml of chloroform and 300 ml of water, and react at 60°C under nitrogen protection After 3 hours, separate the organic phase, cool to 10°C, add 200ml of 1mol / L sodium hydroxide solution to react for 3 hours, collect the organic phase, wash with 200ml of water, then add 200ml of chloroform, 250ml of isopropanol and 40% 130ml of acetaldehyde was stirred and reacted for 16 hours, then 250ml of water was added, stirred for 30 minutes, the organic phase was separated, concentrated under reduced pressure, 100ml of ice water was added, stirred for 3 hours, filtered, and vacuum dried at 45°C to obtain 7-phenylacetamido- 46 g of p-methoxybenzyl 3-(Z-prop-1-enyl)-4-cephalosporanic acid, with a yield of 93.3%.
[0039] (2) Add 136g of phosphorus pentachloride to 625ml of dichloromethane, cool to 15°C, add 45g of pyridine und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com