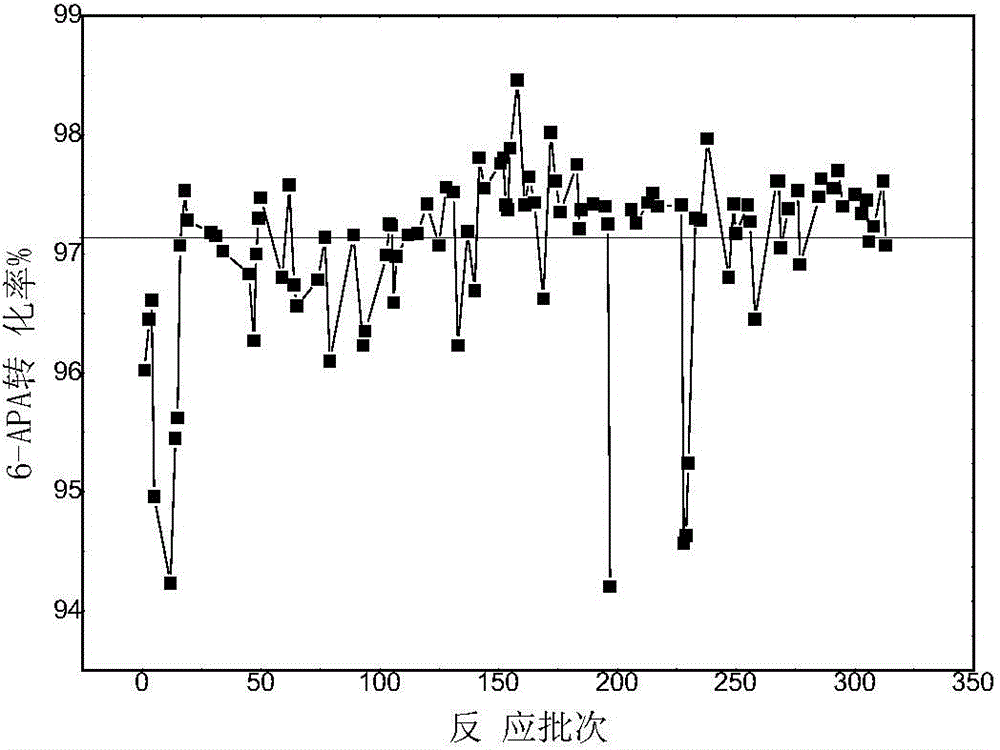
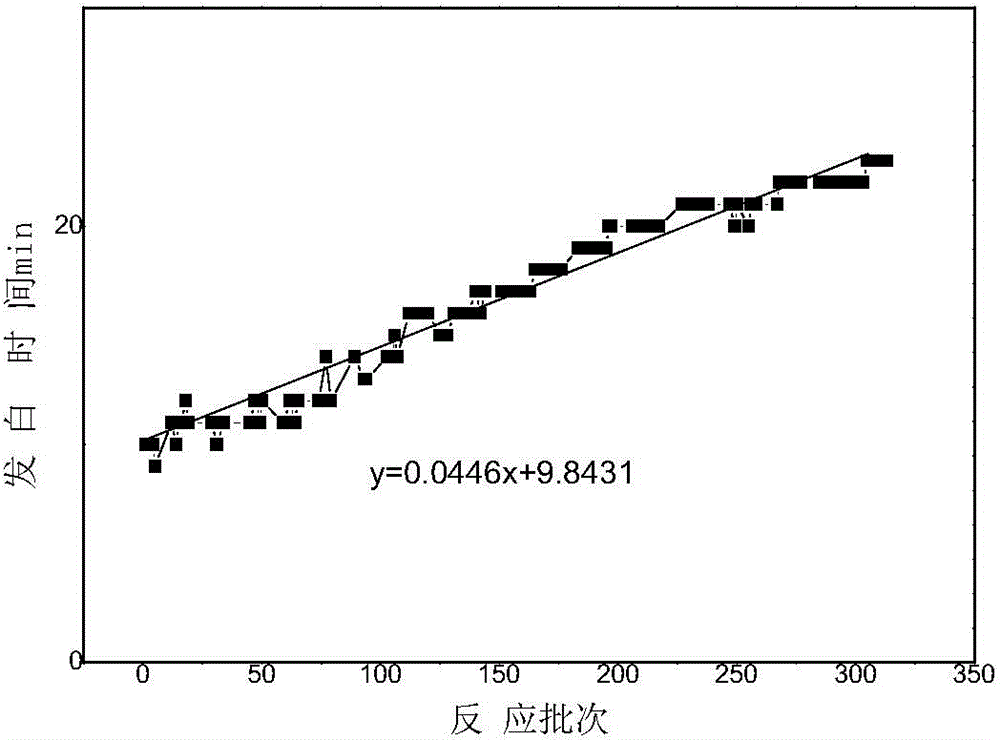
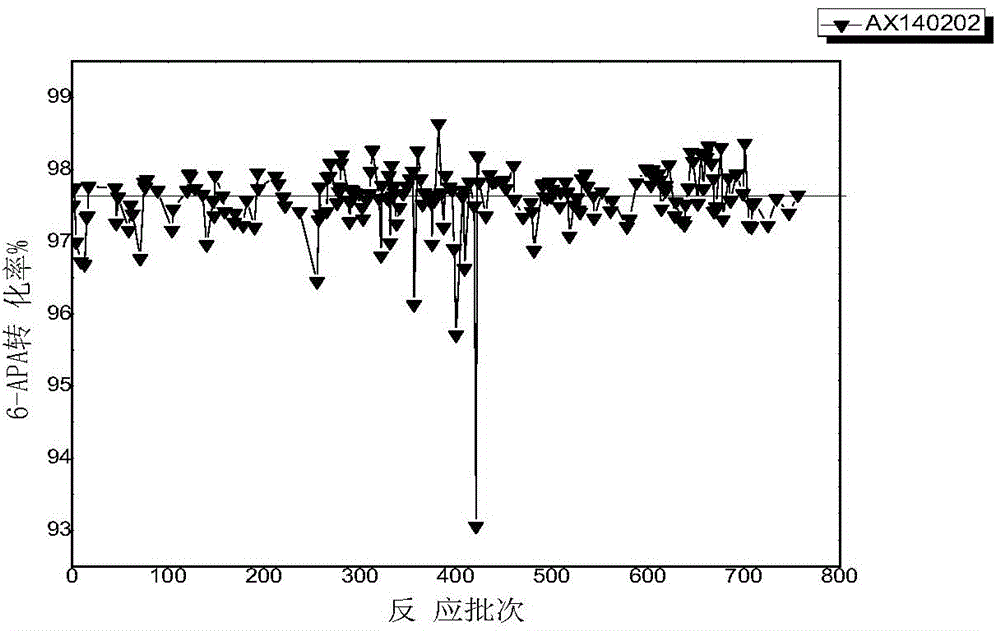
An enzymatic synthesis process of Amoxicillin
A technology of amoxicillin and enzymatic synthesis, which is applied in the field of drug synthesis, can solve the problems of difficult screening and evaluation of immobilized enzymes, cumbersome production process steps, and long reaction time, and achieve good clarity, shortened reaction time, and fast reaction time short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In the reactor, 1300 u of immobilized amoxicillin synthase LK218 was added to 130 mL of deionized water, and the stirring was started. 0.06 mol of 6-APA and 0.0612 mol of D-p-hydroxyphenylglycine methyl ester were added. Sodium hydroxide was added to adjust the reaction pH to 6.3. The reaction temperature was maintained at 19-20°C. Reaction 55min. The reaction solution was separated from the immobilized amoxicillin synthase. The obtained product was dissolved by adjusting the pH value to 0.9-1.1 with hydrochloric acid, filtered, adjusted the pH value with sodium hydroxide and crystallized, and the final pH value was 5.0-5.2. After growing the crystal at 0-5°C for 1 hour, washing and drying, the finished product of amoxicillin is obtained.
Embodiment 2
[0037] In the reactor, 1300 u of immobilized amoxicillin synthase LK218 was added to 130 mL of deionized water, and the stirring was started. 0.06 mol of 6-APA and 0.0618 mol of D-p-hydroxyphenylglycine methyl ester hydrochloride were added. Sodium hydroxide was added to adjust the reaction pH to 6.3. The reaction temperature was maintained at 14-15°C. Reaction 65min. The reaction solution was separated from the immobilized amoxicillin synthase. The obtained product was dissolved by adjusting the pH value to 0.9-1.1 with hydrochloric acid, filtered, adjusted the pH value with sodium hydroxide and crystallized, and the final pH value was 5.0-5.2. After growing the crystal at 0-5°C for 1 hour, washing and drying, the finished product of amoxicillin is obtained.
Embodiment 3
[0039] In the reactor, 1300 u of immobilized amoxicillin synthase LK218 was added to 130 mL of deionized water, and the stirring was started. 0.06 mol of 6-APA and 0.0612 mol of D-p-hydroxyphenylglycine methyl ester were added. Sodium hydroxide was added to adjust the reaction pH to 6.3. The reaction temperature was maintained at 10-11°C. Reaction 70min. The reaction solution was separated from the immobilized amoxicillin synthase. The obtained product was dissolved by adjusting the pH value to 0.9-1.1 with hydrochloric acid, filtered, adjusted the pH value with sodium hydroxide and crystallized, and the final pH value was 5.0-5.2. After growing the crystal at 0-5°C for 1 hour, washing and drying, the finished product of amoxicillin is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com