Method for recycling active ingredients in amoxicillin mother liquor synthesized by enzymatic method
A technology for synthesizing amoxicillin mother liquor by enzymatic method, which is applied in the field of enzymatic synthesis of amoxicillin mother liquor by recycling macroporous resins, can solve the problems of unstable chemical properties, poor product purity, complex components, etc., and avoid adverse reactions , low equipment requirements, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (a) Take 2000L of the mother liquor of amoxicillin synthesized by enzymatic method (among them, the content of residual amoxicillin is 3 mg / mL, the content of D-hydroxyphenylglycine is 15 mg / mL, and the content of D-hydroxyphenylglycine methyl ester is 5 mg / mL). Adjust the pH to 7.5 with sodium hydroxide solution, add 50kg of penicillin acylase, control the temperature at 25°C, and react for 2 hours to hydrolyze the amoxicillin in the mother liquor to generate D-hydroxyphenylglycine and 6-aminopenicillanic acid, D - hydrolysis of p-hydroxyphenylglycine methyl ester to D-p-hydroxyphenylglycine to obtain a hydrolyzate. The content of each component in the hydrolyzate was detected by high performance liquid chromatography, and the content of D-p-hydroxyphenylglycine was 18 mg / mL, and the content of 6-APA was 2 mg / mL.
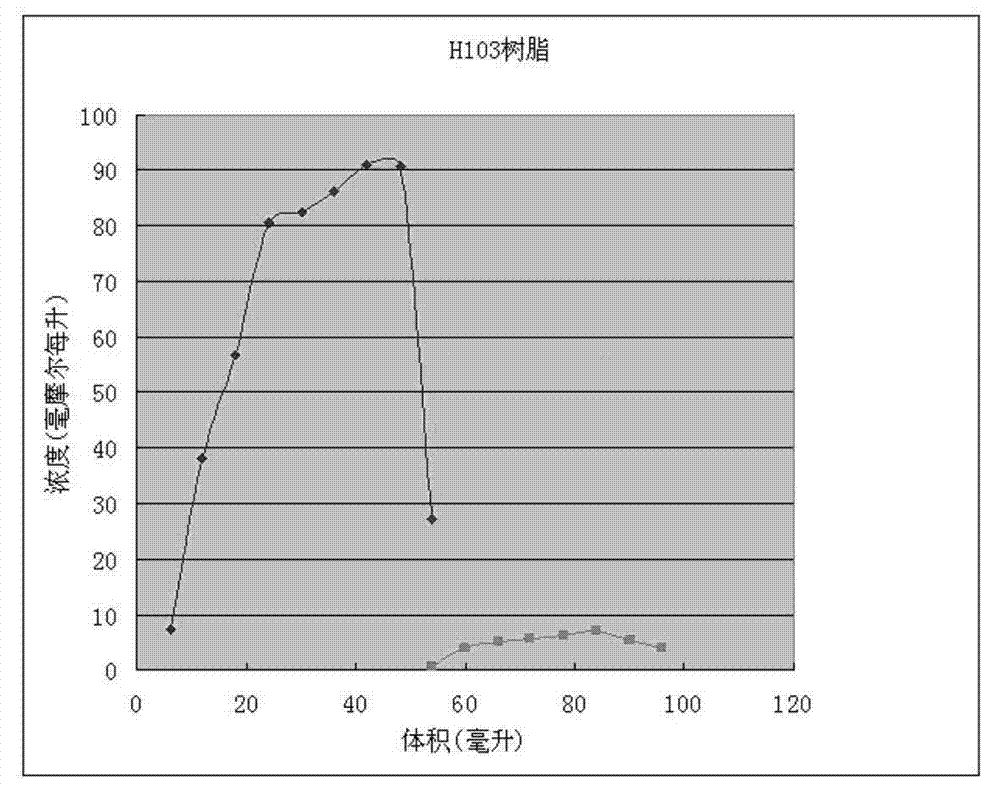
[0034] (b) Use hydrochloric acid with a volume ratio of 15% to adjust the pH of the hydrolyzate to 6.0-7.0, and then separate the hydrolyzate through H103 ...
Embodiment 2
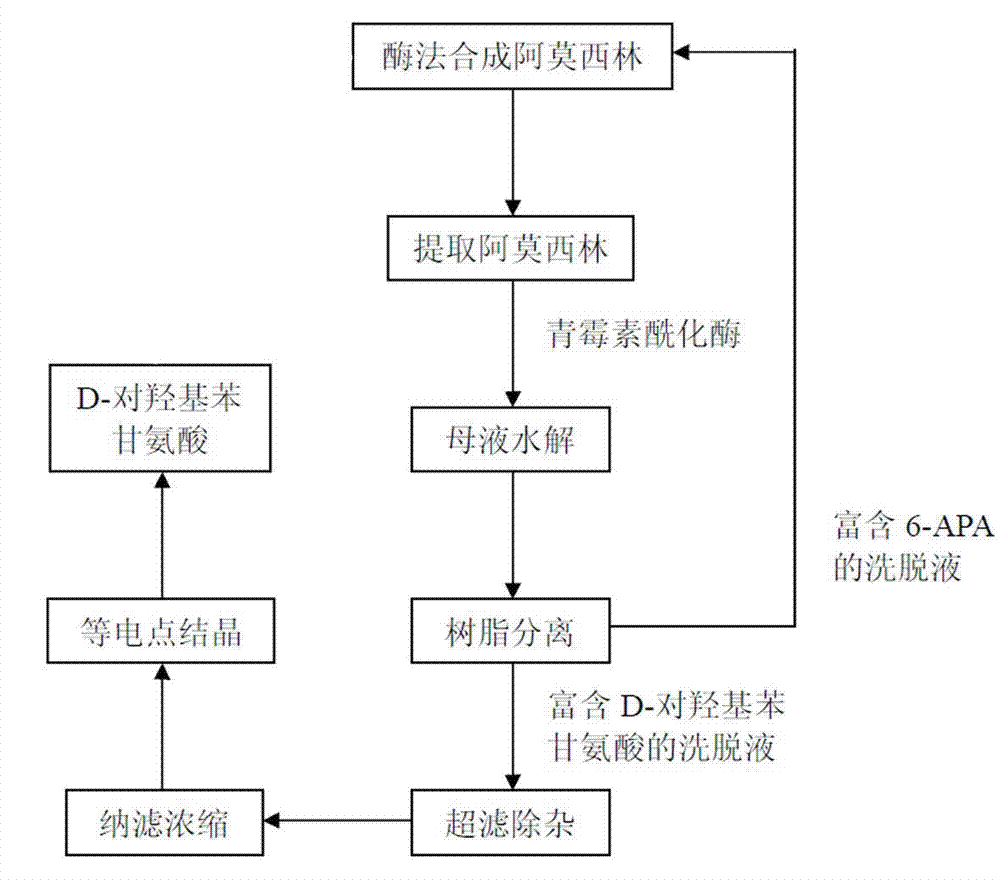
[0042] See the process flow figure 1 , the operation steps are as follows:
[0043] After enzymatically synthesizing amoxicillin, extract amoxicillin, and then recover the mother liquor:
[0044] (a) Mother liquor hydrolysis: Use concentrated ammonia to adjust the pH of the enzymatically synthesized amoxicillin mother liquor (2000L) to 7.5, add penicillin acylase (50kg), react at 30°C for 1 hour, remove the acylase by filtration, and obtain a hydrolyzate ; Under the pH value and temperature conditions, after 1h of reaction, the remaining amoxicillin in the mother liquor is fully hydrolyzed into 6-APA and D-p-hydroxyphenylglycine, and the remaining substrate D-p-hydroxyphenylglycine methyl ester in the synthesis reaction is also hydrolyzed It is D-p-hydroxyphenylglycine.
[0045] (b) Resin separation: Use hydrochloric acid with a volume ratio of 34% to adjust the pH value of the hydrolyzate to 7.0, and use XAD-4 macroporous resin column (produced by Rohn & hass company in the...
Embodiment 3
[0050] (a) Mother liquor hydrolysis: use 10% sodium hydroxide solution by mass ratio to adjust the pH of the enzymatically synthesized amoxicillin mother liquor to 8.0, add penicillin acylase, react at 25°C for 2 h, remove the acylase by filtration, Obtain a hydrolyzate; under the pH value and temperature conditions, after 2 hours of reaction, the residual amoxicillin in the mother liquor is fully hydrolyzed into 6-APA and D-p-hydroxyphenylglycine, and the remaining substrate D-p-hydroxyphenylglycine methyl Esters are also hydrolyzed to D-p-hydroxyphenylglycine.
[0051] (b) Resin separation: use hydrochloric acid with a volume fraction of 15-34% to adjust the pH value of the hydrolyzate to 6.5, and use the XAD1600N macroporous resin column produced by Rohn & hass company in the United States to separate, the sample loading temperature is 18°C, and the flow rate is 1.5BV / h,
[0052] Elute with deionized water, the elution temperature is 30°C, and the flow rate is 3 BV / h. Re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com