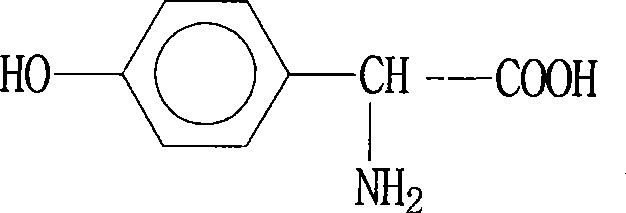
P-hydroxybenzene glycine synthesis technology
A technology for the synthesis of p-hydroxyphenylglycine, which is applied in the preparation of organic compounds, organic chemistry, chemical instruments and methods, etc., and can solve problems such as complicated process operation, increased cost, and HPG color and purity that cannot meet the requirements of separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Example 1: In a 5000L enamel reaction kettle, drop into 740Kg of 40% glyoxylic acid, 580Kg of water, 400Kg of molten phenol, 500Kg of sulfamic acid, 5Kg of p-toluenesulfonic acid, stir and be warmed up to 70° C., keep the reaction for 12 hours, and the reaction is completed, Add 500Kg of water, 20Kg of sodium bisulfite, cool down to 45°C, add ammonia water dropwise, adjust the pH to 4.2 between 2 hours, cool down to 40°C, centrifuge, wash with water until no foam, wash with methanol until the filtrate is colorless, dry Obtained HPG374Kg, content 98.8%, yield 56.0%.
example 2
[0023] Example 2: In a 5000L enamel reaction kettle, put 740Kg of 40% glyoxylic acid, 580Kg of water, 400Kg of molten phenol, 500Kg of sulfamic acid, 5Kg of p-toluenesulfonic acid, stir and heat up to 35°C, and heat the reaction at 35-40°C for 6 hours, then the temperature was raised to 65-70° C. for 8 hours and the reaction was maintained for 8 hours.
example 3
[0024] Example 3: Except that the input glyoxylic acid is self-made 19.3% 1534Kg, other procedures are the same as Example 2, to obtain DL-HPG435Kg, the content (HPLC) is 98.8%, and the yield is 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com