Method for preparing cefprozil in pH responsive regenerative double aqueous phase system
A cefprozil and system technology, which is applied to the preparation of cefprozil and the synthesis field of cefprozil, can solve the problems of high production cost, low conversion rate, long production cycle, etc., and improve the problem of low product conversion rate and molar yield. Improve and avoid high cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] P ADBA / P MDB Synthesis of cefprozil by enzyme reaction phase transfer in two aqueous phase system:
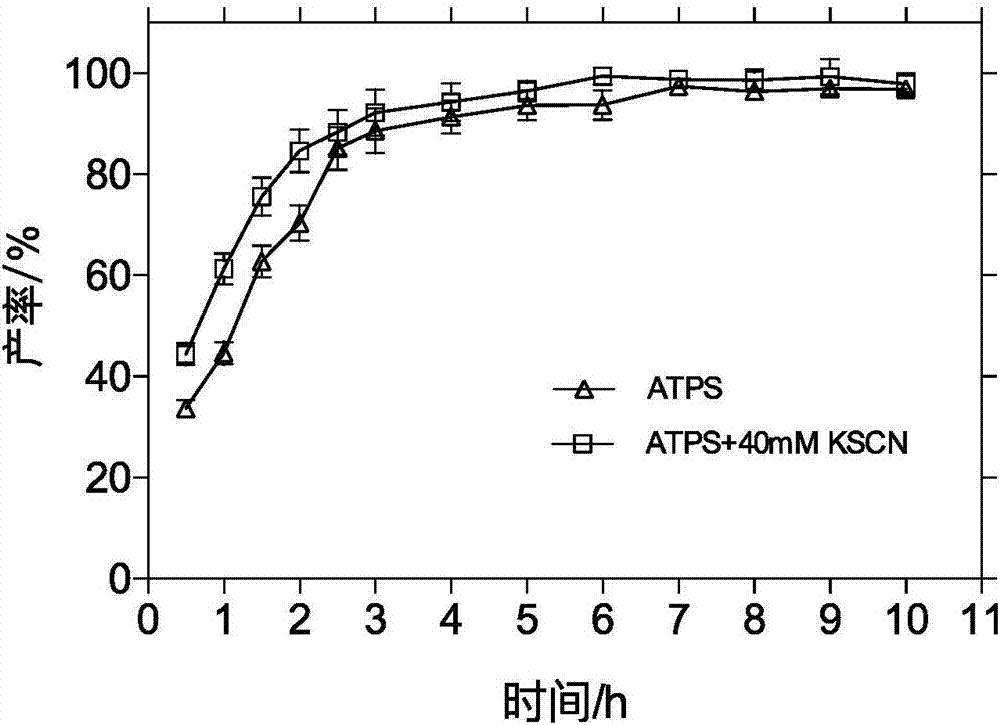
[0052] Configure P ADBA 3%(w / v) / P MDB 3% (w / v) two aqueous phase system 20mL, 0.2700g 7-amino-3-propenyl cephalosporanic acid (7-APRA) and 0.7320g D-p-hydroxyphenylglycine methyl ester hydrochloride (D-HPGME -HCl) into the two-phase system, add 0.0136g of potassium dihydrogen phosphate, adjust the pH of the solution to 5.50, control the reaction temperature to 20°C, add 1g of immobilized penicillin acylase (IPGA), and control the reaction speed to 200r / min, After reacting for 4 hours, the molar yield of cefprozil was 89.41% as detected by high performance liquid chromatography, and the distribution coefficient K of cefprozil remained at 1.30. The pH of the solution was adjusted to reclaim the two-phase polymer, and the recovery rate reached 94.33%. Add 5 times the volume DMF crystallization, centrifugation, washing and drying to obtain the crude product cefprozil. ...
Embodiment 2
[0056] P ADBA / P MDB Synthesis of cefprozil by enzyme reaction phase transfer in two aqueous phase system:
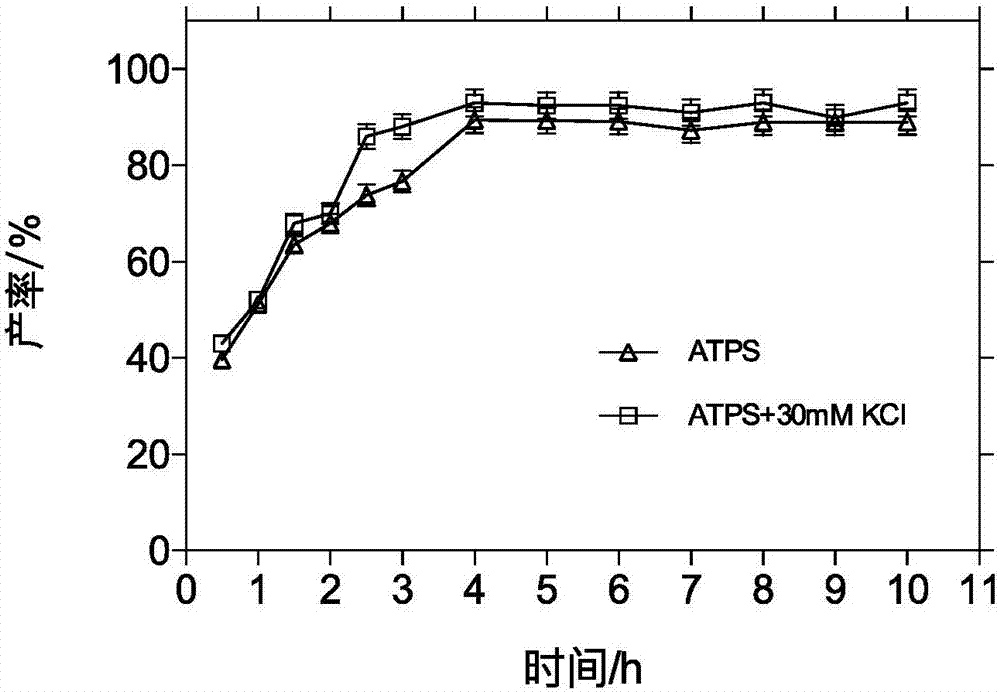
[0057] Configure P ADBA 3%(w / v) / P MDB 3% (w / v) two-phase aqueous system 20mL, add 0.2700g 7-APRA and 0.7320gD-HPGME-HCl to the two-phase aqueous system, add 0.0136g potassium dihydrogen phosphate and 0.0447g potassium chloride, adjust the pH of the solution To 5.50, control reaction temperature is 20 ℃, add 1g IPGA, control reaction speed 200r / min, react 4 hours, cefprozil partition coefficient K remains on 2.13, obtain cefprozil molar yield by liquid phase detection and be 93.02%, improved 16.48%. Adjust the pH of the solution to recover the two-phase polymer, and the recovery rate reaches 94.33%. Add 5 times the volume of DMF to crystallize, centrifuge, wash, and dry to obtain the crude product cefprozil.
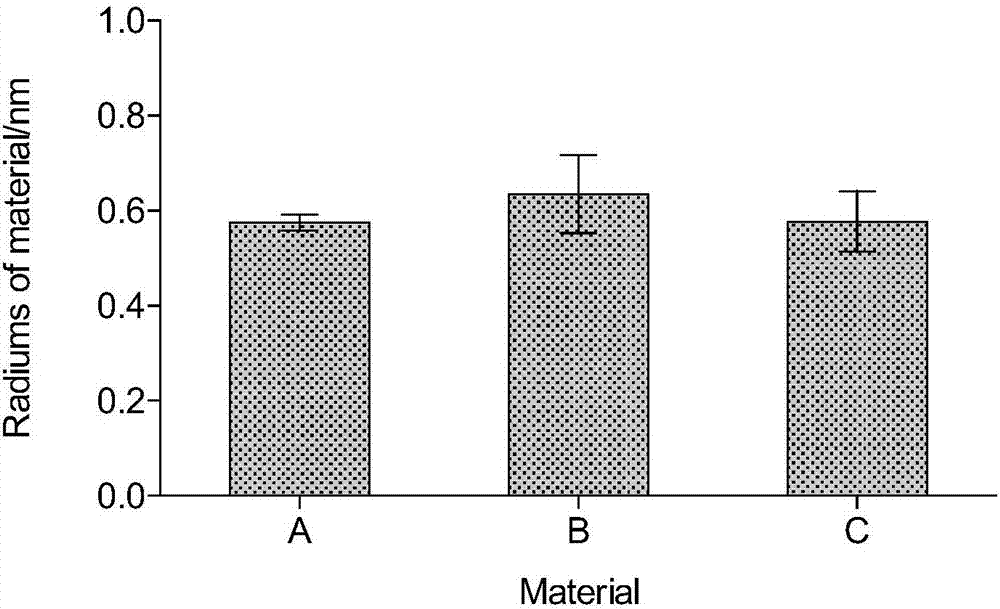
[0058] The particle radius of cefprozil obtained through testing is identical to that of Example 1.
[0059] Such as figure 2 Shown is the P of Examples 1-...
Embodiment 3
[0061] P ADB / P MDB Synthesis of cefprozil by enzyme reaction phase transfer in two aqueous phase system:
[0062] Configure P ADB 4% (w / v) / P MDB 3% (w / v) two-phase aqueous system 20mL, add 0.2831g 7-APRA and 0.3835gD-HPGME-HCl to the two-phase aqueous system, add 0.0136g potassium dihydrogen phosphate, adjust the pH of the solution to 5.40, control the reaction temperature at 20°C, add 1 g of IPGA, control the reaction speed at 200 r / min, and react for 4 hours. The molar yield of cefprozil is 80.19% through liquid phase detection, and the partition coefficient K of cefprozil remains at 1.30. Adjust the pH of the solution to recover the two-phase polymer, and the recovery rate reaches 96.01%. Add 5 times the volume of DMF to crystallize, centrifuge, wash, and dry to obtain the crude product cefprozil.
[0063] The particle radius of cefprozil obtained through testing is identical to that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com