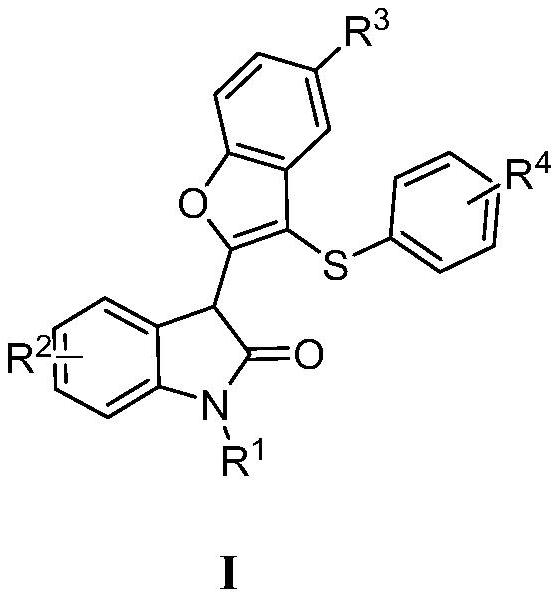
3-thiobenzofuran derivative and synthesis method thereof
A technology of benzofuran and synthesis method, applied in the field of 3-thiobenzofuran derivatives and synthesis thereof, achieves the effects of simple preparation method and operation, good functional group diversity, and improved molar yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of synthetic method of 3-thiobenzofuran derivatives, comprising steps:
[0039]
[0040] For the synthesis method of 3-hydroxy-3-((2-hydroxyphenyl)ethynyl)-1-methylindol-2-one (IIa), please refer to the literature "Org. Biomol. Chem., 2018, 16, 6133" .
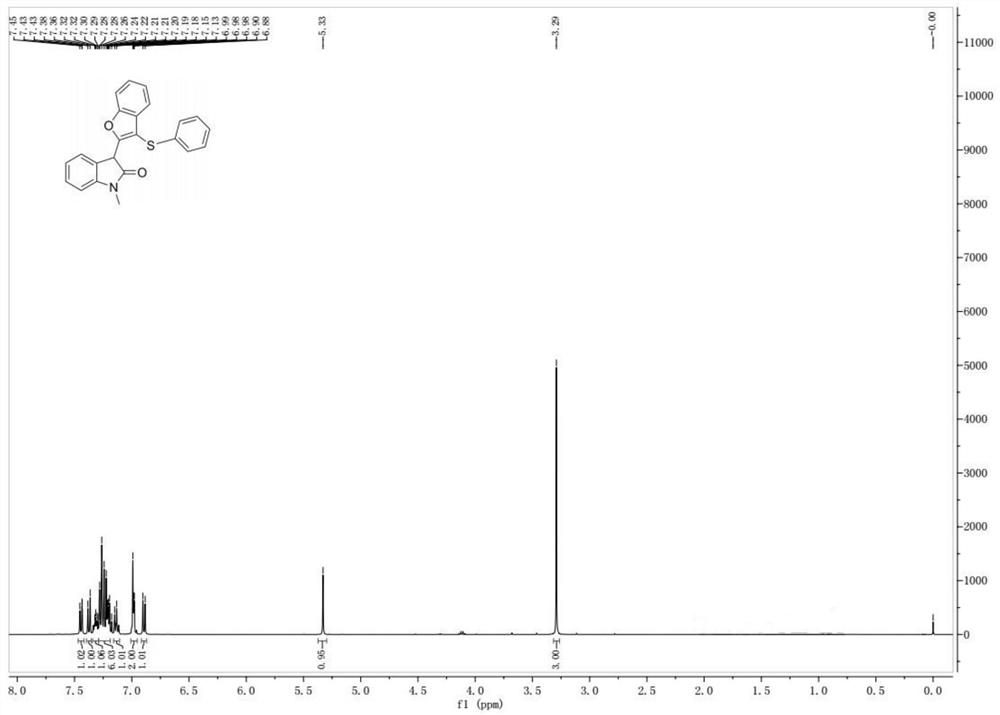
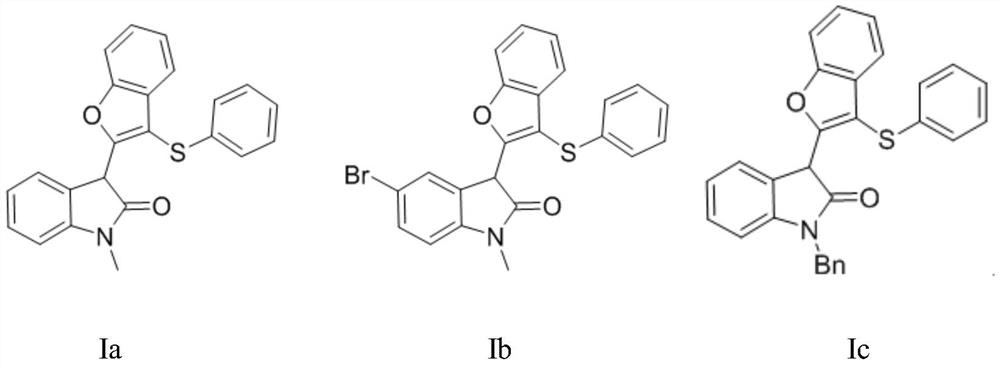
[0041]The preparation steps of compound Ia are: add compound IIa (27.9mg, 0.1mmol) in 10mL round bottom flask, methylene chloride 2ml, thiophenol IIIa (13.2mg, 0.12mmol), add boron trifluoride diethyl ether ( 17mg, 0.12mmol), reacted at 25°C for 12 hours under the protection of nitrogen. Separation by silica gel column chromatography (eluent: petroleum ether (60-90° C.) / ethyl acetate=8:1, V:V) gave light yellow solid Ia (32.3 mg, molar yield 87%) with a purity greater than 95%. The target product was confirmed by NMR and high-resolution mass spectrometry.
[0042] Characterization data of compound Ia: light yellow solid, melting point 197-198°C;
[0043] NMR spectrum such as figure 1 As shown, the NMR da...
Embodiment 2
[0046] A kind of synthetic method of 3-thiobenzofuran derivatives, comprising steps:
[0047]
[0048] For the synthesis method of compound IIb, please refer to the literature "Org. Biomol. Chem., 2018, 16, 6133".
[0049] The preparation steps of compound Ib are: add compound IIb (35.7mg, 0.1mmol) in 10mL round bottom flask, methylene chloride 2ml, thiophenol IIIa (13.2mg, 0.12mmol), add boron trifluoride diethyl ether ( 17mg, 0.12mmol), reacted at 25°C for 12 hours under the protection of nitrogen. Separation by silica gel column chromatography (eluent: petroleum ether (60-90° C.) / ethyl acetate=8:1, V:V) gave light yellow solid Ib (38.2 mg, molar yield 85%) with a purity greater than 95%. The target product was confirmed by NMR and high-resolution mass spectrometry.
[0050] Characterization data of compound Ib: light yellow solid, melting point 220-221°C;
[0051] The NMR data are as follows:
[0052] 1 H NMR (400MHz, CDCl3) δ7.47(d, J=7.7Hz, 1H), 7.44–7.36(m, 2H),...
Embodiment 3
[0054] A kind of synthetic method of 3-thiobenzofuran derivatives, comprising steps:
[0055]
[0056] For the synthesis method of compound IIc, please refer to the literature "Org. Biomol. Chem., 2018, 16, 6133"
[0057] The preparation steps of compound Ic are: add compound IIc (35.5mg, 0.1mmol) in 10mL round bottom flask, dichloromethane 2ml, thiophenol IIIa (13.2mg, 0.12mmol), add boron trifluoride diethyl ether ( 17mg, 0.12mmol), reacted at 25°C for 12 hours under the protection of nitrogen. Separation by silica gel column chromatography (eluent: petroleum ether (60-90° C.) / ethyl acetate=8:1, V:V) gave light yellow solid Ib (40.2 mg, molar yield 90%) with a purity greater than 95%. The target product was confirmed by NMR and high-resolution mass spectrometry.
[0058] Characterization data of compound Ic: pale yellow solid, melting point 242-243°C;
[0059] 1 H NMR (400MHz, CDCl 3 )δ7.45(d,J=7.3Hz,1H),7.42–7.35(m,3H),7.30(dt,J=13.6,5.6Hz,6H),7.26–7.18(m,4H),7.18–...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com