Process for direct preparation of amoxicillin by liquid 6-APA (amino penicillanic acid)
A technology of 6-APA and amoxicillin, which is applied in the field of drug preparation, can solve problems such as not being suitable for expanding production, increasing 6-APA degradation, and affecting yield, so as to improve drug safety, reduce investment, Effect of reducing energy loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
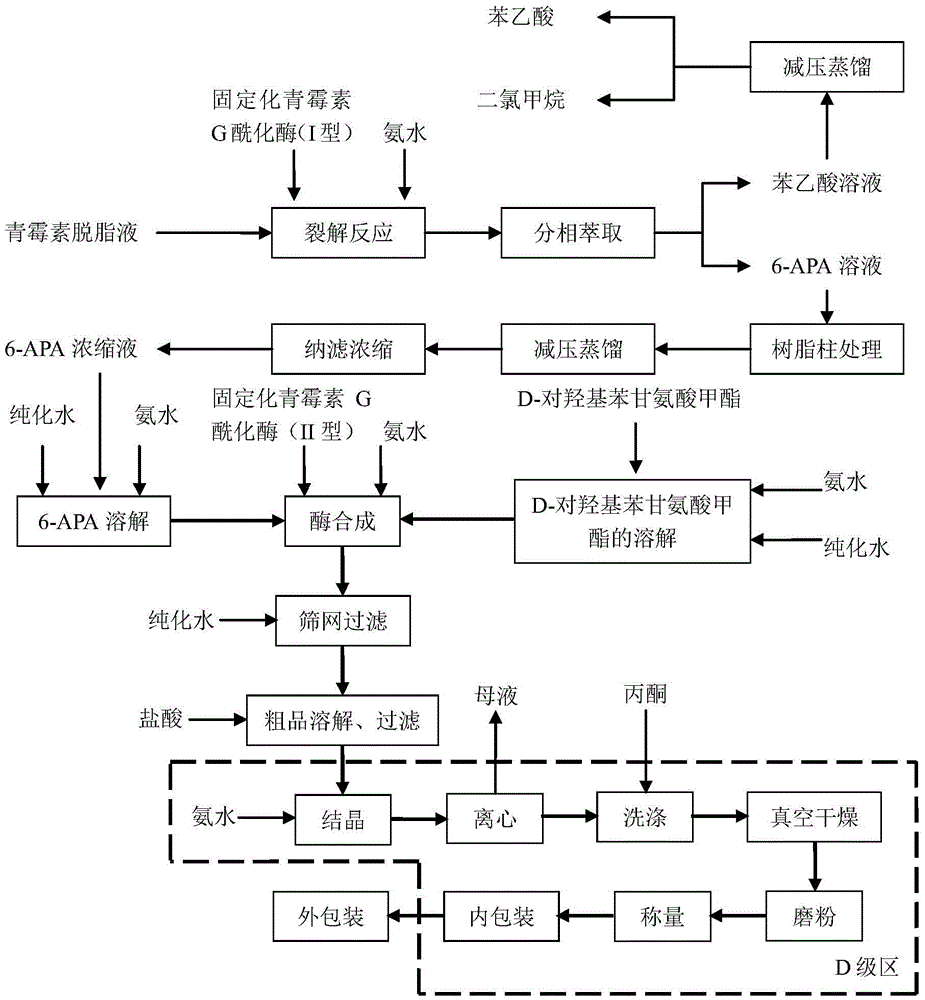
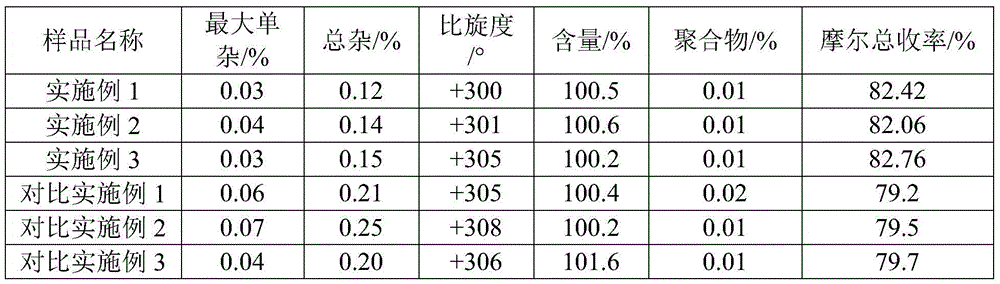
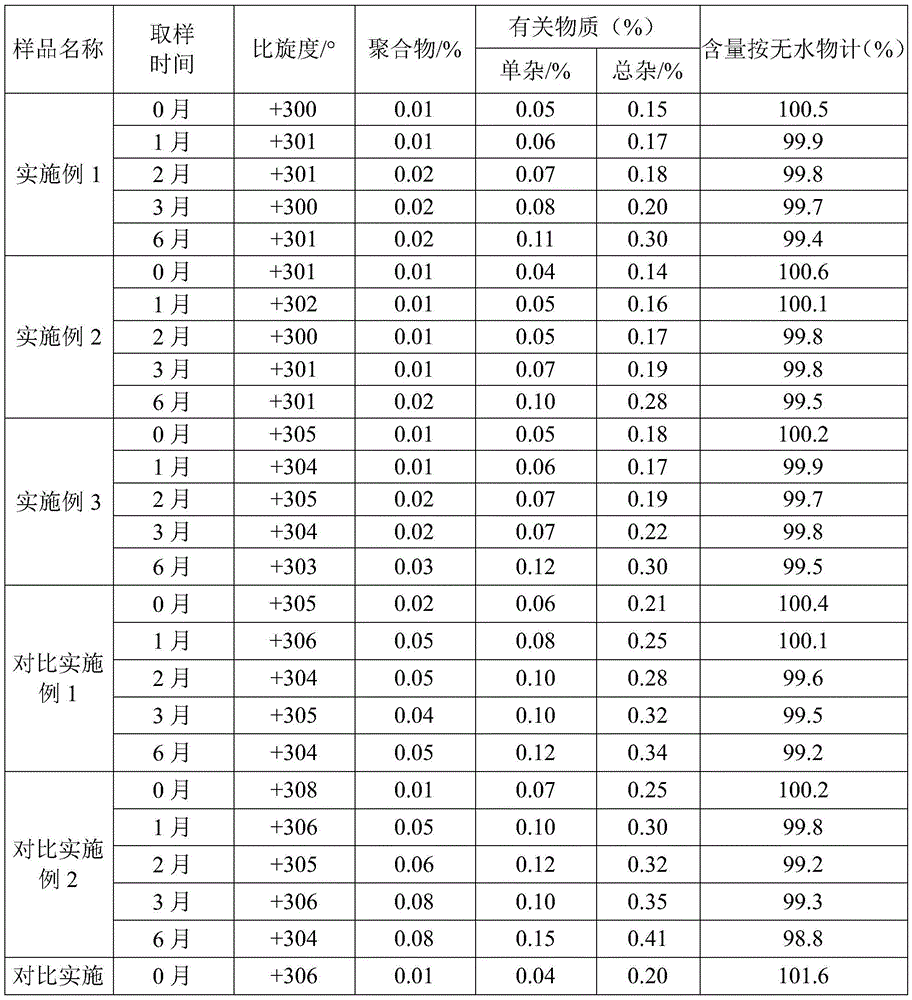
[0022] Example 1 Liquid 6-APA straight-through amoxicillin
[0023] (1) in the reactor that immobilized penicillin G acylase (I type) 1kg is housed, add penicillin concentration and be 20kg of penicillin degreasing liquid of 10wt%, control cleavage reaction pH to be 8.0, temperature 30 ± 1 ℃, when pH has no When fluctuating, detect the content of penicillin, calculate when the conversion rate of penicillin is 98.8%, discharge, obtain the mixed cracking solution of 6-APA and phenylacetic acid;
[0024] (2) Add 50% dichloromethane of the volume of the lysate to the lysate, and adjust the pH value to 1.0 with 30wt% hydrochloric acid, stir, leave to separate phases, and separate to obtain an aqueous phase containing 6-APA;
[0025] (3) Chromatographically separate the aqueous phase through a LXT-080 macroporous adsorption resin column, control the flow rate to 4BV / h, and the temperature at 5-10°C. After passing through the resin column, it is detected that the residual phenylaceti...
Embodiment 2
[0029] Example 2 Liquid 6-APA straight-through amoxicillin
[0030] (1) in the reactor that immobilized penicillin G acylase (I type) 2.4kg is housed, add penicillin concentration and be 15wt% penicillin degreasing liquid 20kg, control cleavage reaction pH to be 7.8, temperature 28 ± 1 ℃, when pH When there is no fluctuation, the content of penicillin is detected, and when the conversion rate of penicillin is calculated to be 98.2%, the material is discharged to obtain a mixed lysis solution of 6-APA and phenylacetic acid;
[0031] (2) Add dichloromethane with 100% volume of the lysate to the lysate, and adjust the pH value to 1.0 with 30wt% hydrochloric acid, stir, leave to separate phases, and separate to obtain an aqueous phase containing 6-APA;
[0032] (3) Chromatographically separate the aqueous phase through a LXT-080 macroporous adsorption resin column, control the flow rate to 3BV / h, and the temperature at 5-10°C. After passing through the resin column, it is detected...
Embodiment 3
[0036] Example 3 Liquid 6-APA straight-through amoxicillin
[0037] (1) in the reactor that immobilized penicillin G acylase (I type) 22kg is housed, adding penicillin concentration is 20kg of penicillin degreasing liquid of 18wt%, and the control cleavage reaction pH is 8.2, and temperature 32 ± 1 ℃, when pH has no When fluctuating, detect the content of penicillin, calculate when the conversion rate of penicillin is 98.3%, discharge, obtain the mixed lysis solution of 6-APA and phenylacetic acid;
[0038] (2) Add dichloromethane of 1 / 3 of the volume of the lysate to the lysate, and adjust the pH value to 1.0 with 30wt% hydrochloric acid, stir, stand still and separate phases, and separate to obtain an aqueous phase containing 6-APA;
[0039](3) Chromatographically separate the aqueous phase through a LXT-081 macroporous adsorption resin column, control the flow rate to 5BV / h, and the temperature at 5-10°C. After passing through the resin column, it is detected that the resid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com