A method for synthesizing d-p-hydroxyphenylglycine methyl ester
A technology for p-hydroxyphenylglycine methyl ester and p-hydroxyphenylglycine, which is applied in the field of synthesizing D-p-hydroxyphenylglycine methyl ester and can solve the problems of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
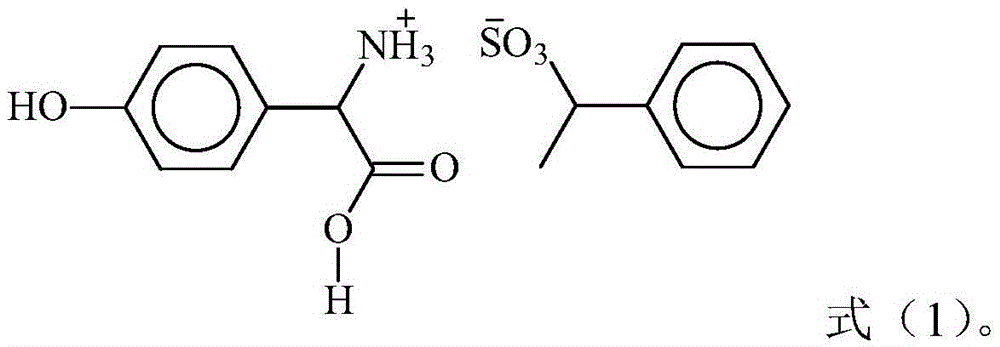
[0018] The D-p-hydroxyphenylglycine resolving agent salt can be obtained commercially, or can be prepared according to various methods known to those skilled in the art. According to a preferred embodiment of the present invention, the D-p-hydroxyphenylglycine resolving agent salt is obtained by mixing DL-p-hydroxyphenylglycine and sodium phenylethanesulfonate in hydrochloric acid and salicylaldehyde at 100-110°C. Reaction in the presence of 8-12 hours to obtain. In the preparation process, relative to 100 parts by weight of sodium phenylethanesulfonate, the amount of DL-p-hydroxyphenylglycine can be 75-90 parts by weight, and the amount of hydrochloric acid can be 50-60 parts by weight, The amount of the salicylic acid can be 2-3 parts by weight; preferably, relative to 100 parts by weight of sodium phenylethanesulfonate, the amount of DL-p-hydroxyphenylglycine is 80-85 parts by weight, the The amount of hydrochloric acid is 53-56 parts by weight, and the amount of salicylal...
specific Embodiment approach
[0026] In the present invention, the method for removing salicylaldehyde in step (3) is not particularly limited, and various existing methods can be used to carry out. According to a specific embodiment of the present invention, salicylaldehyde is removed by the following method: add water to the reaction product obtained after the reaction, and then evaporate water to entrain and remove salicylaldehyde. The specific operation is a technology in the art It is well-known to the personnel and will not be described in detail here.
[0027] The present invention will be described in detail below by way of examples.
[0028] In the following examples and comparative examples:
[0029] In step (1), the yield of D-p-hydroxyphenylglycine methyl phenylethanesulfonate=the actual output of D-p-hydroxyphenylglycine methyl phenylethanesulfonate ÷ D-p-hydroxyphenylglycine methyl benzene Theoretical yield of ethyl ethanesulfonate × 100%.
[0030] The yield of D-p-hydroxyphenylglycine methy...
Embodiment 1
[0032] This embodiment is used to illustrate the method for synthesizing D-p-hydroxyphenylglycine methyl ester provided by the present invention.
[0033] (1) Esterification:
[0034] Add 11700mL of methanol into a 30L glass reactor, start stirring, take 6000g of D-p-hydroxyphenylglycine phenylethanesulfonate (also known as DD salt, the same below) into the glass reactor, and control the temperature below 25 2230 g of thionyl chloride was added dropwise at °C. After the dropwise addition of the thionyl chloride, the temperature was raised to a reflux state, and the reflux was maintained for 3 hours. After the esterification reaction was completed, an esterification liquid was obtained. Detected by HPLC, the yield of D-p-hydroxyphenylglycine methyl phenylethanesulfonate in the esterified solution was 98.5%.
[0035] (2) Neutralization:
[0036] Add water 2000mL and D-hydroxyphenylglycine methyl ester 50g (as seed crystal) in 3L glass reaction bottle, control temperature at 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com