Improved method for preparing amoxicillin by enzymic method
A technology for amoxicillin and enzymatic preparation, applied in the field of amoxicillin preparation, can solve the problems of poor fluidity, high toxicity to operators, low bulk density and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
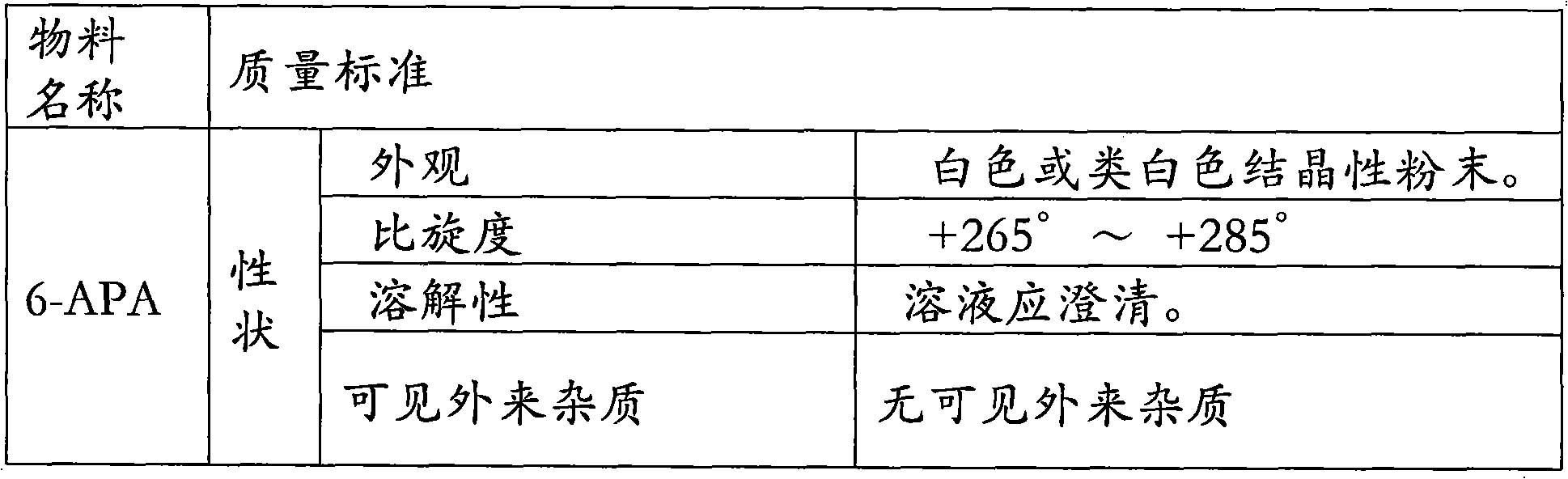
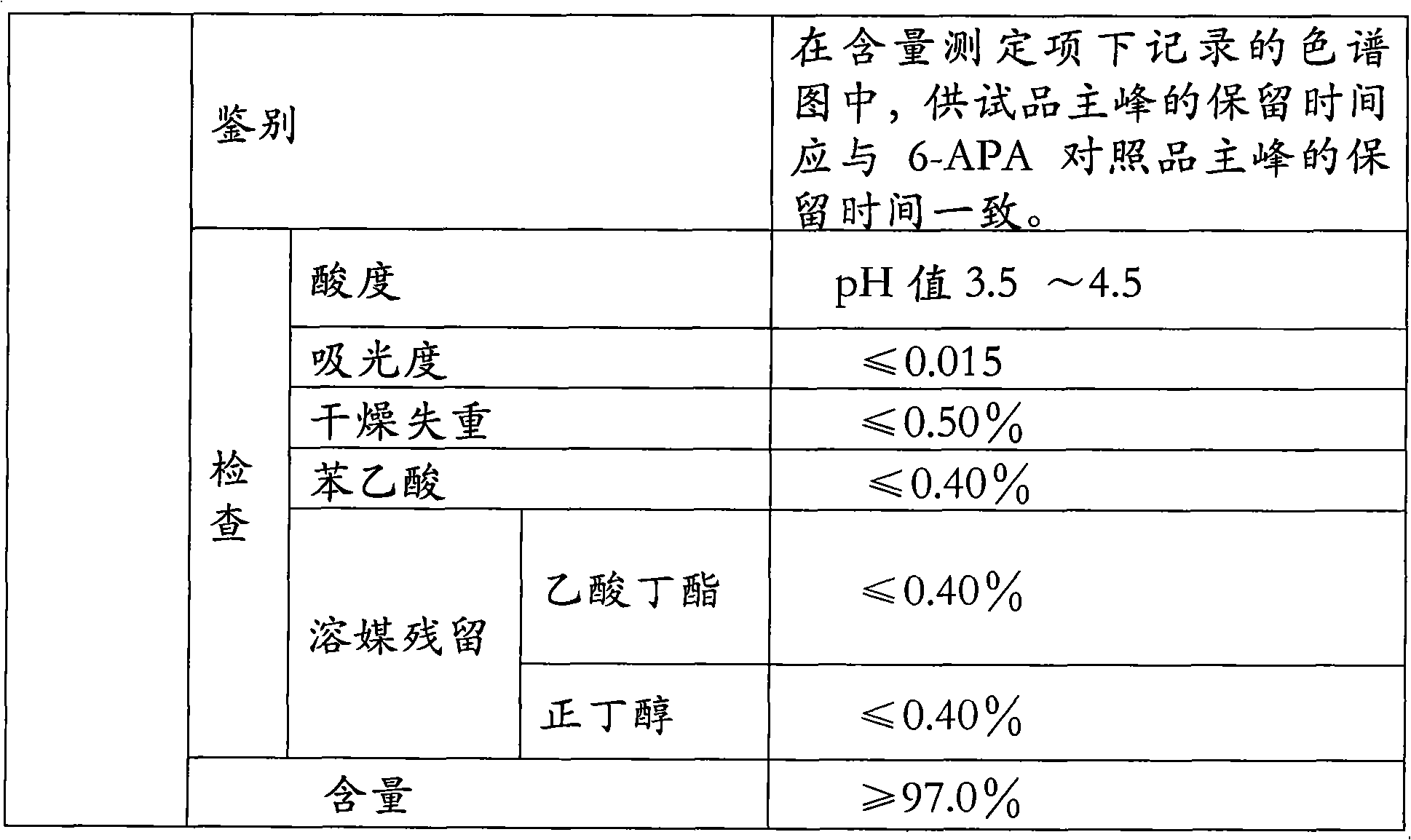
[0091] Material preparation:
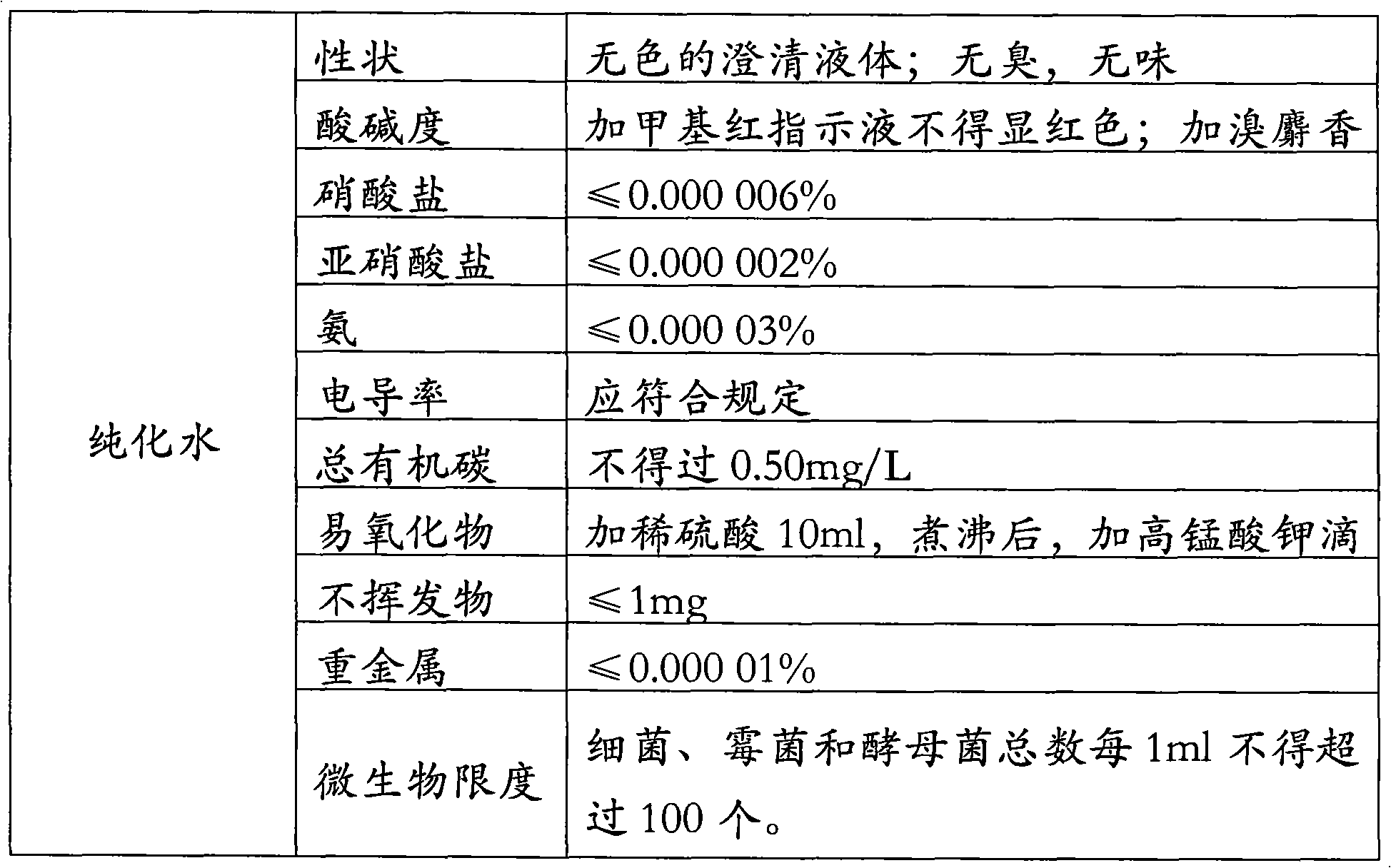
[0092] 6-APA 12g; D-p-hydroxyphenylglycine methyl ester hydrochloride 15.6g; penicillin G acylase 2.2KU / L; 3mol / l ammonia 8ml; acetone 60ml (90% concentration); 6mol / l hydrochloric acid 11ml; 6mol / l ammonia water: 9.5ml; purified water 500ml.
[0093] Preparation Process:
[0094] Add 240ml of purified water into a 1000ml beaker, add 12g of 6-APA while stirring, then add dropwise 3mol / l ammonia water to adjust the pH to 7.5, keep the temperature at 15°C, 6-APA is completely dissolved, and the amount of 3mol / l ammonia water is 5ml; add to the beaker Add D-p-hydroxyphenylglycine methyl ester hydrochloride; then add penicillin G acylase to start the reaction; keep the reaction system at a constant temperature of 22°C, add ammonia water to control the pH=6.25, and use 3ml of 3mol / l ammonia water; after 1 hour of reaction , taking samples at regular intervals and measuring the 6-APA conversion rate (mg / ml) by HPLC: 23.17 in the first hour; 18.11 in ...
Embodiment 2
[0096] Material preparation:
[0097] 6-APA 12g; D-p-hydroxyphenylglycine methyl ester hydrochloride 16.8g; penicillin G acylase 2.2KU / L; 3mol / l ammonia 9ml; acetone 60ml (98% concentration); 6mol / l hydrochloric acid 12ml; 6mol / l ammonia water 10ml; purified water: 500ml
[0098] Preparation Process:
[0099] 240ml of purified water was added to a 1000ml beaker, and 12g of 6-APA was added while stirring. Add 3 mol / l ammonia water dropwise, when T=15°C and pH=7.5, 6-APA is completely dissolved, and the dosage of 3 mol / l ammonia water is 5ml. Add D-p-hydroxyphenylglycine methyl ester hydrochloride to the beaker. Add synthetase and start the reaction. Keep the reaction system at a constant temperature of 22°C, add ammonia water to control the pH=6.25, and use 4ml of 3mol / l ammonia water. After reacting for 1 hour, sample 6-APA at regular intervals to measure the conversion rate (mg / ml): 1st hour: 22.89; 2nd hour: 17.55; 3rd hour: 8.68; 4th hour: 6.71; 5th hour: 4.33; stop t...
Embodiment 3
[0102] Material preparation:
[0103] 6-APA 12g; D-hydroxyphenylglycine methyl ester hydrochloride 19.2g; penicillin G acylase 2.2KU / L; 3mol / l ammonia 10.5ml; methanol 60ml (85% concentration); 6mol / L hydrochloric acid 12.5 ml; 6mol / L ammonia water 11ml; purified water 500ml.
[0104] Preparation Process:
[0105] Add 240ml of purified water into a 1000ml beaker, then add 12g of 6-APA, and stir at the same time; add dropwise 3mol / l ammonia water, when T=15°C, pH=7.5, 6-APA is completely dissolved, and the dosage of 3mol / l ammonia water is 6ml; Add D-p-hydroxyphenylglycine methyl ester hydrochloride into the beaker; add synthetase to start the reaction; keep the reaction system at a constant temperature of 22°C, add ammonia water to control the pH=6.25, and use 4.5ml of 3mol / l ammonia water; - APA conversion rate (mg / ml): 23.01 in the 1st hour; 17.61 in the 2nd hour; 8.54 in the 3rd hour; 6.83 in the 4th hour; 4.35 in the 5th hour; stop the reaction; Crude.
[0106] In a 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com