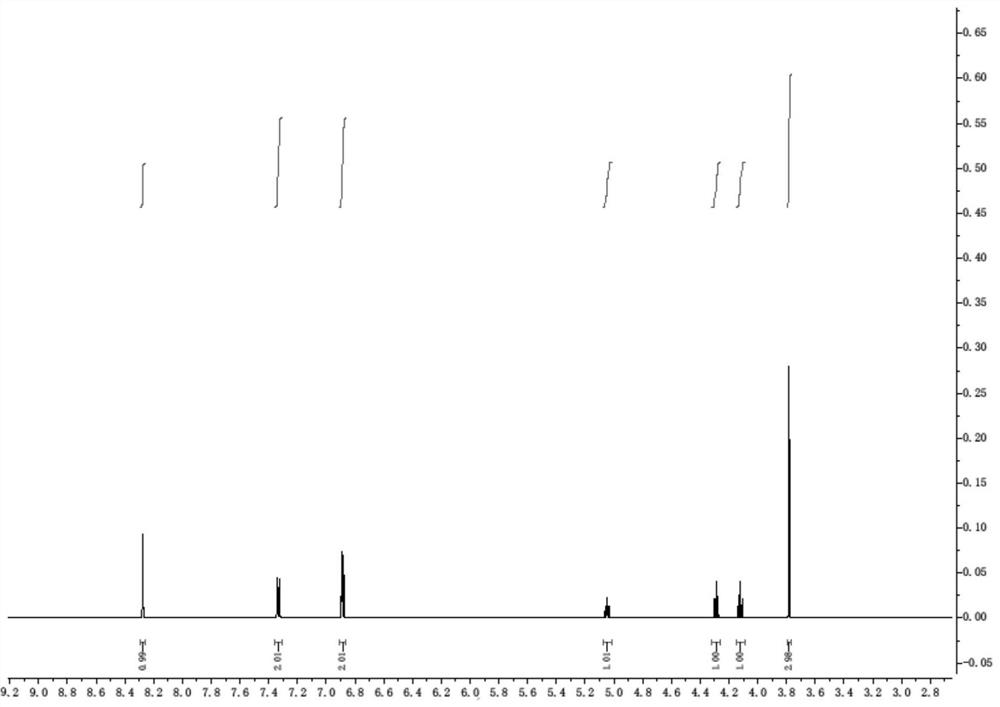
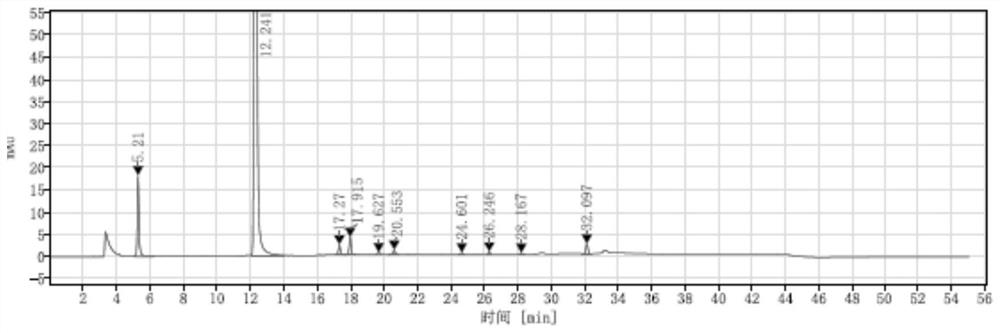
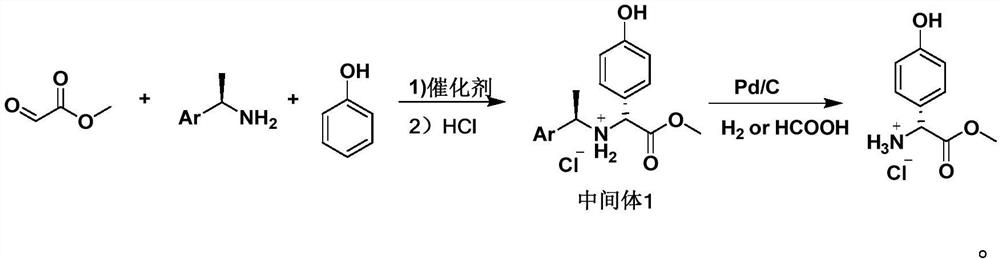
Preparation method of D-p-hydroxyphenylglycine methyl ester hydrochloride suitable for industrial production
A technology of p-hydroxyphenylglycine methyl ester and hydrochloride, which is applied in the field of preparation of D-p-hydroxyphenylglycine methyl ester hydrochloride, can solve the problems of high raw material and production costs, long production cycle, and low production efficiency. Achieve the effects of avoiding the use of splitting agents, improving production efficiency, reducing costs and three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 intermediate 1
[0038]
[0039] In a 1L three-necked flask, add 500 milliliters of toluene, then 60 milliliters of toluene solution of methyl glyoxylate (50% concentration, 0.3 mol) and 36.4 grams of S-phenylethylamine (0.3 mol), and stir at 20 to 30 ° C for 2 Then add 5.2 grams (0.03mol) of p-toluenesulfonic acid (0.03mol) and 33.8 grams of phenol (0.36mol), and stir for 12 hours at 20~30°C. After the reaction finishes, add 150 milliliters of hydrogen chloride methanol solution (10%), and heat to 70-80°C, then lower the temperature to 20-30°C, filter with suction to obtain intermediate 1, and dry it in vacuum at 50°C for 8 hours to obtain 70.3 g of solid, with a yield of 73%.
Embodiment 2
[0040]The synthesis of embodiment 2 intermediate 1
[0041]
[0042] In a 1L three-neck flask, add 500 ml of dichloromethane, then 60 ml of toluene solution of methyl glyoxylate (50% concentration, 0.3 mol) and 36.4 g of S-phenylethylamine (0.3 mol), at 20 to 30 °C Stir for 2 hours, then add 2.9 grams of methanesulfonic acid (0.03mol) and 33.8 grams of phenol (0.36mol), and stir for 12 hours at 20 to 30°C. After the reaction is over, add 150 milliliters of hydrogen chloride ethanol solution (10%), and heat to reflux, then lower the temperature to 20-30°C, filter with suction to obtain intermediate 1, and dry it in vacuum at 50°C for 8 hours to obtain 59.7 g of solid, with a yield of 62%.
Embodiment 3
[0043] The synthesis of embodiment 3 intermediate 1
[0044]
[0045] In a 1L three-necked flask, add 500 ml of xylene, then 60 ml of toluene solution of methyl glyoxylate (50% concentration, 0.3 mol) and 36.4 g of S-phenylethylamine (0.3 mol), and stir at 20 to 30 ° C 2 hours, then add 3.4 grams (0.03mol) of trifluoroacetic acid and 33.8 grams of phenol (0.36mol), stir at 20~30 ℃ for 12 hours, after the reaction finishes, add 150 milliliters of hydrogen chloride methanol solution (10%), heat to 70-80°C, then lower the temperature to 20-30°C, filter with suction to obtain intermediate 1, and vacuum-dry at 50°C for 8 hours to obtain 73.2 g of solid, with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com