A method for analyzing d(-) p-hydroxyphenylglycine content in d(-) p-hydroxyphenylglycine methyl ester
A technology of p-hydroxyphenylglycine methyl ester and p-hydroxyphenylglycine, which is applied in the field of pharmaceutical, chemical, and pharmaceutical testing, can solve problems such as affecting yield and quality, poor precision, and undetermined analysis methods, and achieves high sensitivity and accurate results. , good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
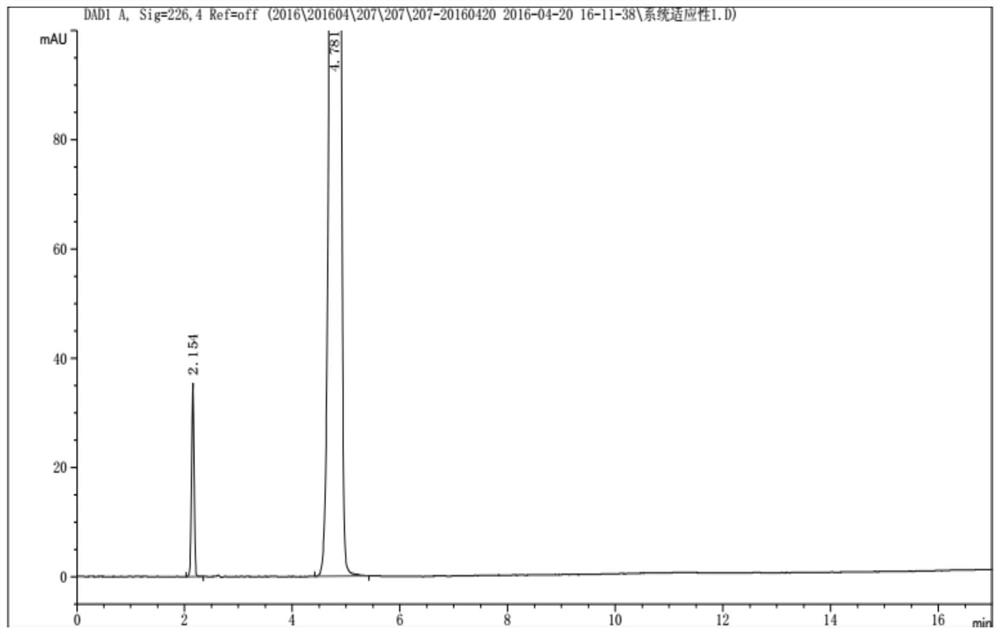
[0045] Under selected chromatographic conditions: variable wavelength ultraviolet detector, detection wavelength: 226nm; flow rate: 1.5ml / min; column temperature: 25°C; injection volume: 5μl; chromatographic column: Ultimate XB-Phenyl(II);
[0046] Buffered saline solution: 10mM potassium dihydrogen phosphate + 10mM triethylamine hydrochloride (solvent is water), adjust pH to 3.5 with phosphoric acid.
[0047] Mobile phase A: buffered saline solution: acetonitrile = 98:2, mobile phase B: buffered saline solution: acetonitrile = 80:20. Elute with gradient ratios. The gradient elution program is shown in the table below:
[0048] time (min) 0 3 15 17 Mobile phase A, % 100 100 0 0 Mobile phase B, % 0 0 100 100
[0049] D(-)p-Hydroxyphenylglycine stock solution: Weigh 50 mg of D(-)p-Hydroxyphenylglycine reference substance into a 100ml volumetric flask, add 0.1% phosphoric acid to dissolve to volume, and shake well.
[0050]System suitability...
Embodiment 2
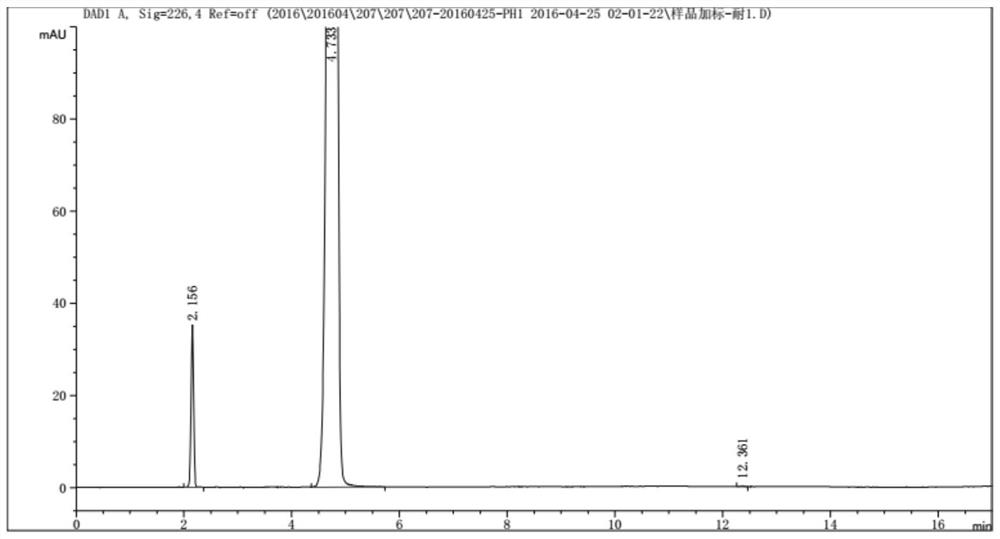
[0054] Under selected chromatographic conditions: variable wavelength ultraviolet detector, detection wavelength: 226nm; flow rate: 1.5ml / min; column temperature: 25°C; injection volume: 5μl; chromatographic column: Ultimate XB-Phenyl(II);
[0055] Buffered saline solution: 10mM potassium dihydrogen phosphate + 10mM triethylamine hydrochloride, adjust the pH to 3.5±0.2 with phosphoric acid.
[0056] The mobile phase is mobile phase A: buffered saline solution: acetonitrile = 98:2, mobile phase B: buffered saline solution: acetonitrile = 80:20. Elute with gradient ratio, add 1 needle of system adaptability solution, p-hydroxyphenylglycine / p-hydroxyphenylglycine methyl ester tailing factor ≤ 2.0, resolution of p-hydroxyphenylglycine and the nearest peak is not less than 1.5; enter the spiked sample For 2 injections of the solution, the absolute difference of the test results of the spiked sample solution requires p-hydroxyphenylglycine ≤ 0.1%, and the largest single unknown impu...
Embodiment 3
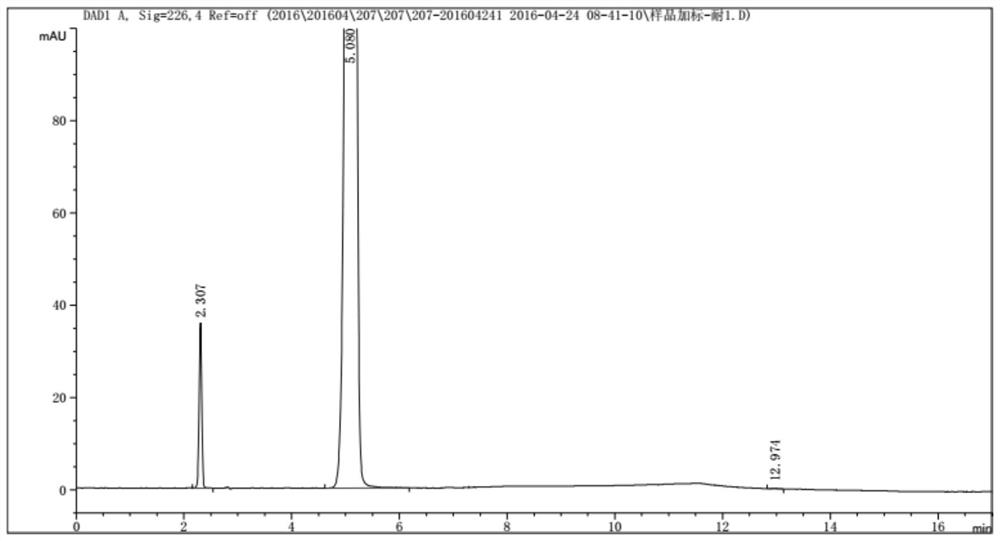
[0064] Under selected chromatographic conditions: variable wavelength ultraviolet detector, detection wavelength: 226nm; flow rate: change the flow rate to 1.4ml / min and 1.6ml / min. Column temperature: 25°C; Injection volume: 5μl; Chromatographic column: Ultimate XB-Phenyl(II);
[0065] Buffered saline solution: 10mM potassium dihydrogen phosphate + 10mM triethylamine hydrochloride, adjust the pH to 3.5 with phosphoric acid.
[0066] The mobile phase is mobile phase A: buffered saline solution: acetonitrile = 98:2, mobile phase B: buffered saline solution: acetonitrile = 80:20. Inject 1 injection of system adaptability solution, D(-) p-hydroxyphenylglycine / D(-) p-hydroxyphenylglycine methyl ester tailing factor ≤ 2.0, and the resolution of D(-) p-hydroxyphenylglycine and the nearest peak is not less than 1.5, 2 injections of the sample spiked solution; the absolute difference of the detection results of the spiked test solution requires D(-) p-hydroxyphenylglycine ≤ 0.1%, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com