Process for synthesizing lornoxicam intermediate against inflammation and pain
An anti-inflammatory analgesic, lornoxicam technology, applied in the field of compound synthesis, can solve the problems of low total yield, long reaction route, high raw material price, etc., and achieve the goal of reducing the discharge of three wastes, reducing risks, and low consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
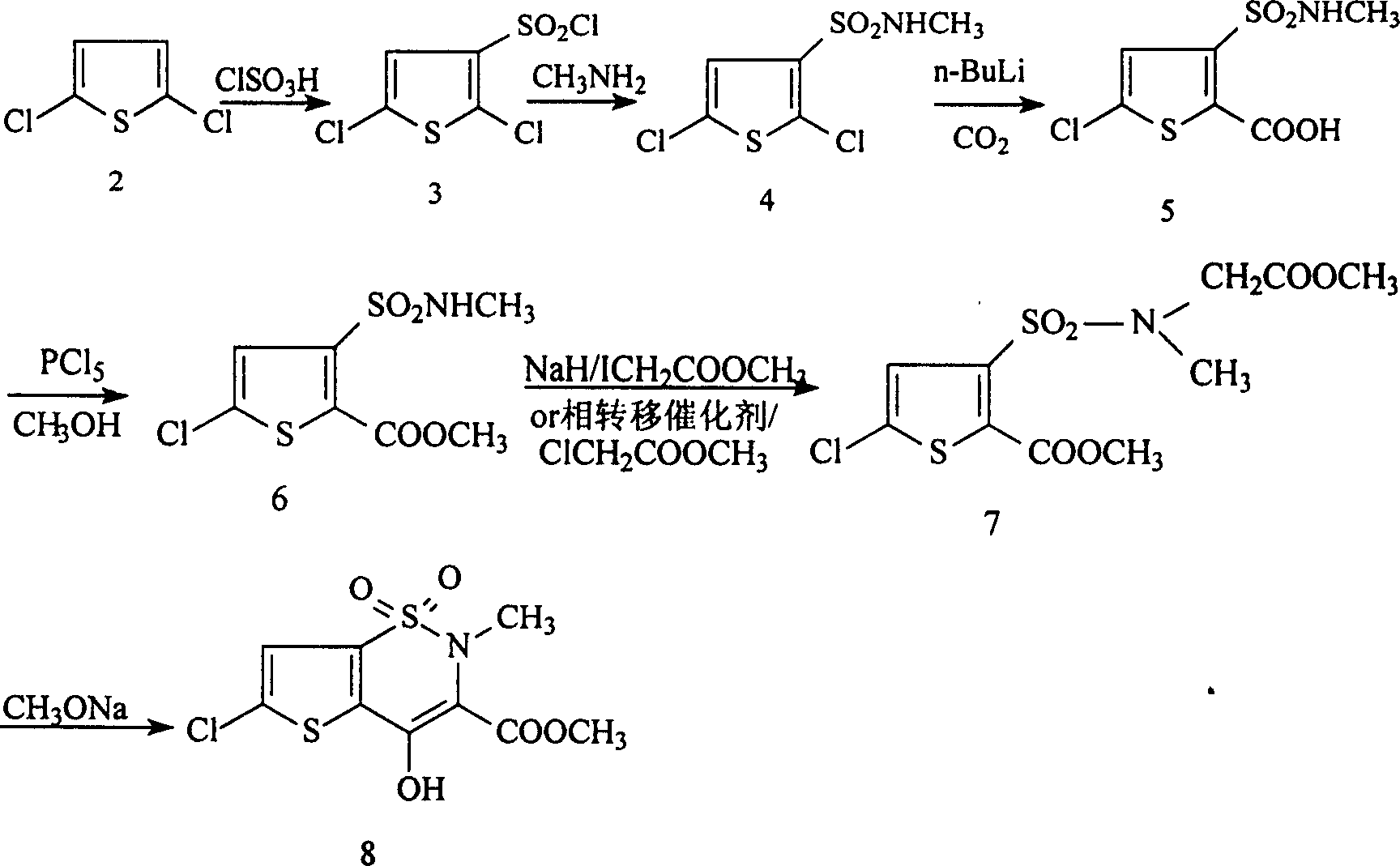
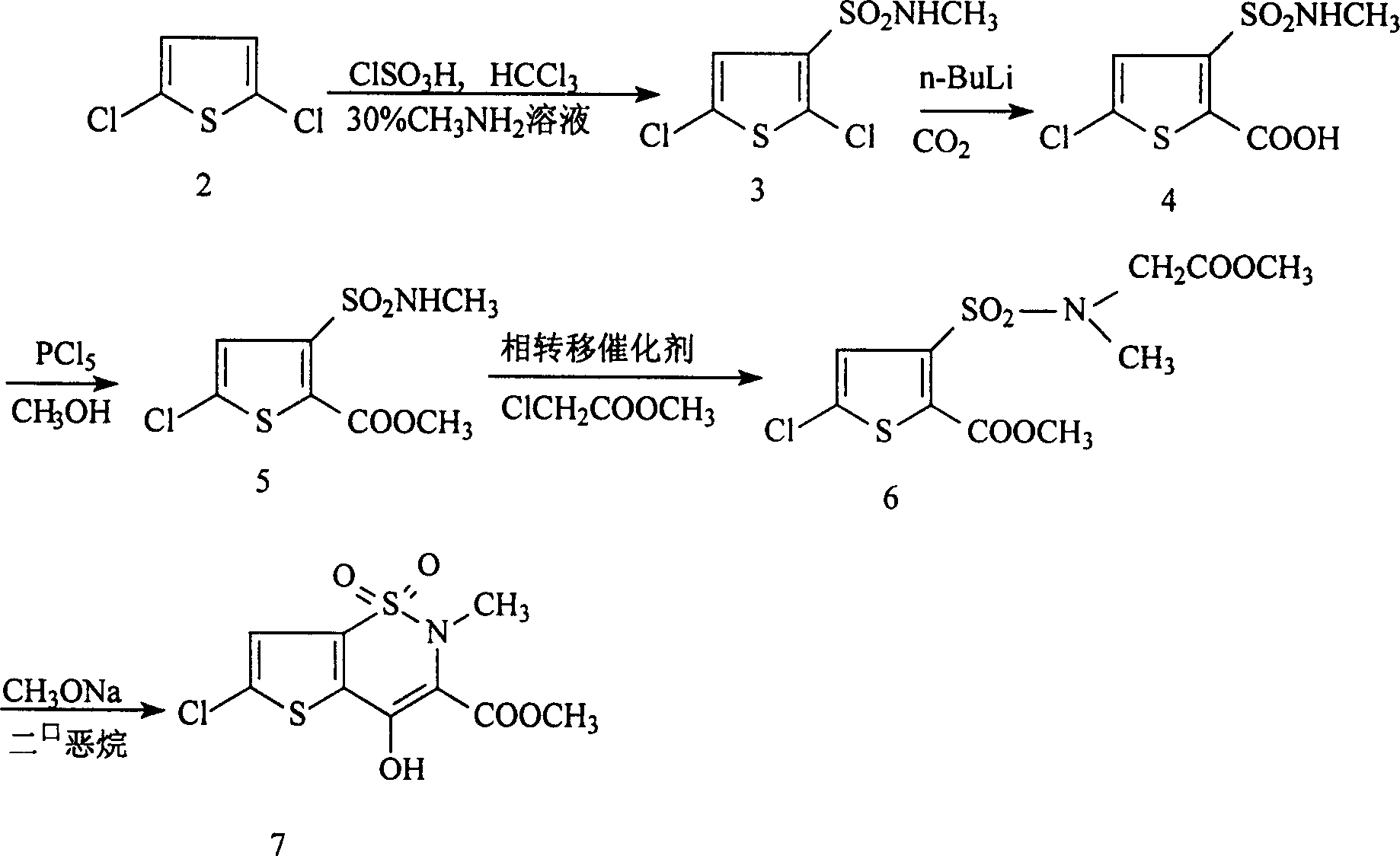
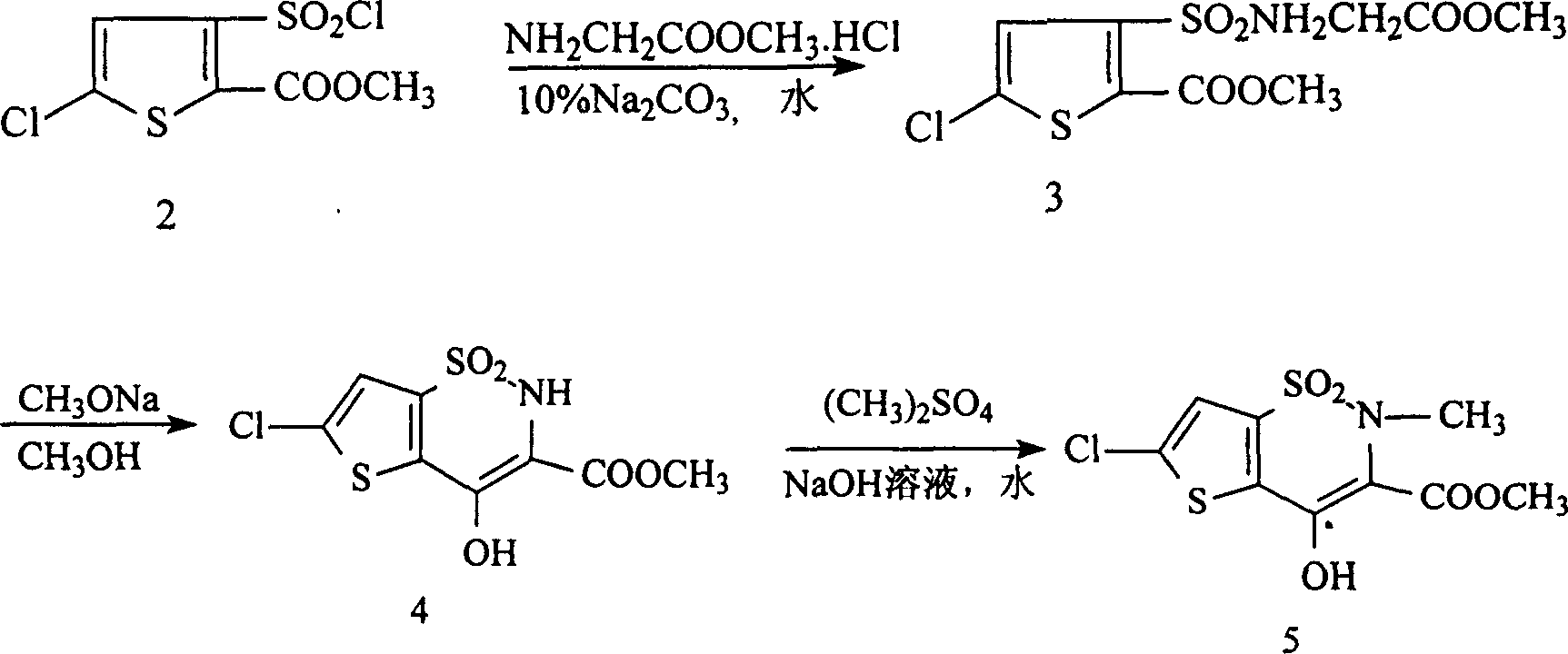
[0016] (1). Synthesis of methyl 5-chloro-3-sulfonamidoacetate thiophene-2-carboxylate:
[0017] Add 10.3g of methyl 5-chloro-3-chlorosulfonylthiophene-2-carboxylate and 30g of methanol into a 250ml four-necked flask. At the same time, 54.5g of 8% sodium carbonate aqueous solution and 18g of 26% glycine methyl ester hydrochloride aqueous solution were added dropwise, and after the addition was completed, they were incubated at 15°C for 10 hours. Then, it was filtered and dried to obtain 12 g of methyl 5-chloro-3-sulfonamidoacetate thiophene-2-carboxylate, with a melting point of 117-118.5° C. and a yield of 95.8%.
[0018] (2). Synthesis of 6-chloro-4-hydroxyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxylic acid methyl ester-1,1-dioxide:
[0019] 12.5 g of methyl 6-chloro-3-sulfonamidoacetate thiophene-2-carboxylate and 74 g of 8% sodium methoxide in methanol were reacted at 65° C. for 8 hours. Cool to room temperature, then filter to obtain 6.7g 6-chloro-4-hydroxyl-2H-thieno[2,3-...
Embodiment 2
[0023] (1). Synthesis of methyl 5-chloro-3-sulfonamidoacetate thiophene-2-carboxylate:
[0024] Add 10.3g of methyl 5-chloro-3-chlorosulfonylthiophene-2-carboxylate and 30g of methanol water into a 250ml four-necked flask. At the same time, 69g of 6% sodium carbonate aqueous solution and 47g of 10% glycine methyl ester hydrochloride aqueous solution were added dropwise, and after the addition was completed, they were incubated at 15°C for 20 hours. Then, it was filtered and dried to obtain 11.8 g of methyl 5-chloro-3-sulfonamidoacetate, thiophene-2-carboxylate, with a yield of 94.2%.
[0025] (2). Synthesis of 6-chloro-4-hydroxyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxylic acid methyl ester-1,1-dioxide:
[0026] 12.5 g of methyl 6-chloro-3-sulfonamidoacetate thiophene-2-carboxylate and 40 g of 16% sodium methoxide in methanol were reacted at 71° C. for 4 hours. Cool to room temperature, then filter to obtain 7g 6-chloro-4-hydroxyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxylic ac...
Embodiment 3
[0030] (1). Synthesis of methyl 5-chloro-3-sulfonamidoacetate thiophene-2-carboxylate:
[0031] Add 10.3g of methyl 5-chloro-3-chlorosulfonylthiophene-2-carboxylate and 30g of methanol into a 250ml four-necked flask. At the same time, 125g of 3% sodium carbonate aqueous solution and 80g of 6% glycine methyl ester hydrochloride aqueous solution were added dropwise, and after the addition was completed, the mixture was kept at 15° C. for 10 hours. Then it was filtered and dried to obtain 11.2 g of methyl 5-chloro-3-sulfonamidoacetate thiophene-2-carboxylate with a yield of 89.4%.
[0032] (2). Synthesis of 6-chloro-4-hydroxyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxylic acid methyl ester-1,1-dioxide:
[0033] 12.5 g of methyl 6-chloro-3-sulfonamidoacetate thiophene-2-carboxylate and 120 g of 6% sodium methoxide in methanol were reacted at 40° C. for 15 hours. Cool to room temperature, then filter to obtain 6.3g of 6-chloro-4-hydroxyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxylic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com