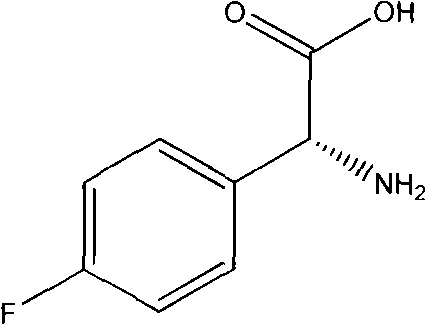
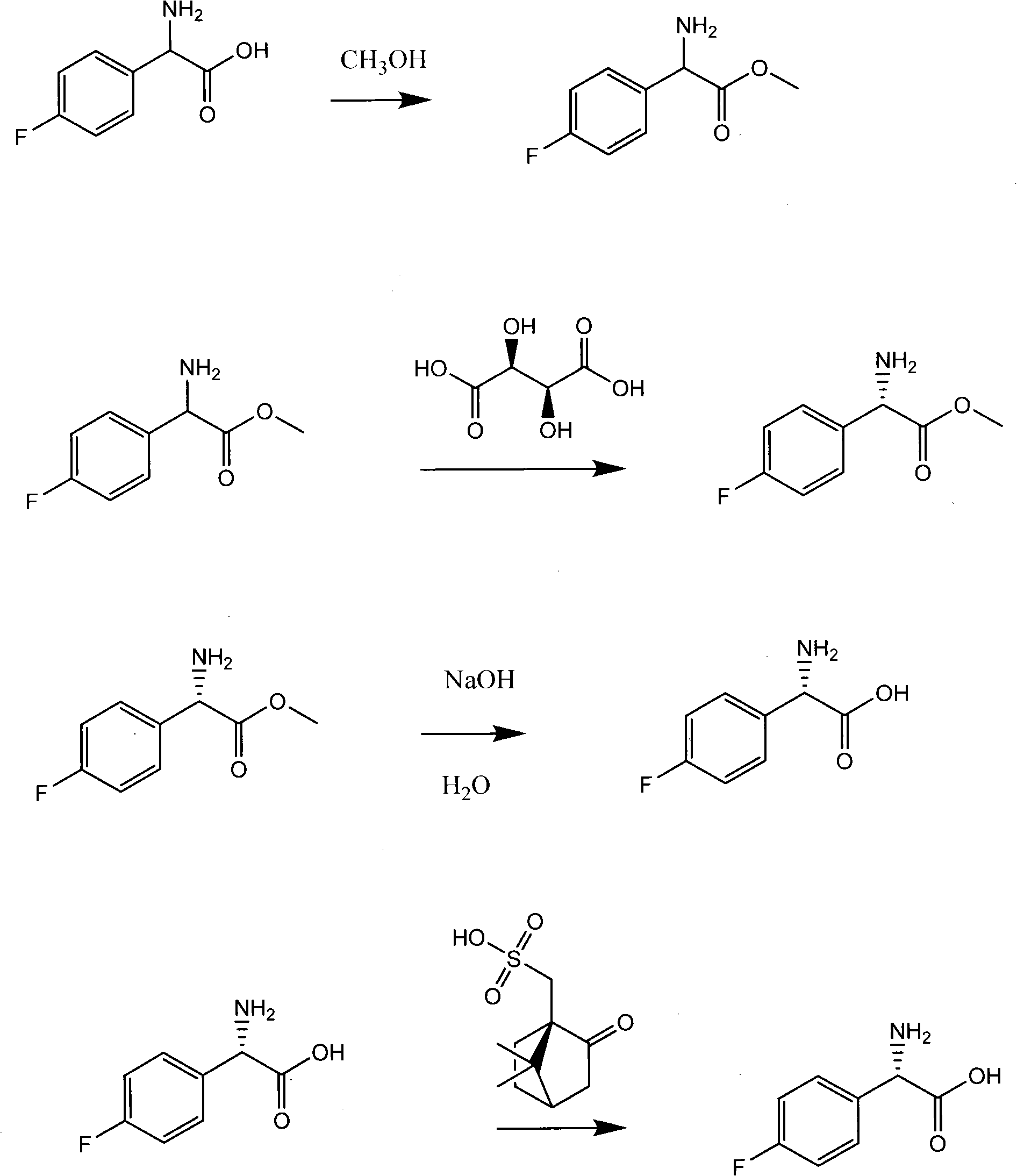
Preparation method of L(+)-p-fluorophenyl glycine
A technology of p-fluorophenylglycine and fluorophenylglycine methyl ester, which is applied in the field of L-camphorsulfonic acid as a refined preparation, can solve the problems of high cost and complex route process, and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Synthesis of p-fluorophenylglycine methyl ester
[0031] In a dry 2000 ml four-necked flask, add 170 grams of DL-p-fluorophenylglycine and 1000 grams of methanol. At room temperature, add 147 grams of concentrated sulfuric acid dropwise, keeping the temperature at 35-40 ° C. After adding the sulfuric acid, heat and reflux for 4 hour, then reclaim methanol, first normal pressure recovery to about 100 ° in the bottle temperature, stop recovery, then continue to add methanol, repeat esterification, reclaim methanol once, (in the recovery process, there may be products that evaporate together with methanol, resulting in product Yield drops). Cool to below 40°, add 320 grams of cold ice water, then drop concentrated ammonia water to neutralize, keep the temperature in the bottle at about 40°, neutralize to PH8-8.5, add 80 grams of toluene, stir for 5 minutes, static layer, water layer Extract again with a little toluene and separate (sometimes there may be a lot of unreacte...
example 2
[0033] Synthesis of L-tartrate double salt of L-fluorophenylglycine methyl ester
[0034] DL-p-fluorophenylglycine methyl ester: 184 grams
[0035] Tartaric acid: (dextrorotatory) 166 grams
[0036] Absolute ethanol: 1150 grams
[0037] p-Fluorobenzaldehyde 18g
[0038] Add 184 grams of methyl esters, 166 grams of tartaric acid, 1150 grams of absolute ethanol, and 18 grams of p-fluorobenzaldehyde in a dry 2000 milliliter four-necked flask, heat and reflux for 10 minutes, then cool and crystallize, stir for 24 hours, (normal temperature) suction filtration, Wash the filter bottle with an appropriate amount of absolute ethanol until it becomes white, drain it, combine the mother liquor and washing liquor, and wait for the next feeding for later use. Note that it must be operated in an anhydrous condition, otherwise the mother liquor will not be usable for the second time and will be reconstituted. 205 grams of salt, enter the next step of hydrolysis, double salt [α] 20 D =5...
example 3
[0040] Separation of mother liquor and treatment of p-fluorophenylglycine methyl ester
[0041] If the split mother liquor is used for splitting, use it three more times, and after the water content is determined to be greater than 1, the mother liquor should be evaporated to remove ethanol to recover p-fluorophenylglycine methyl ester. Heated in methanol solution for decolorization, and evaporated to dryness for later use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com