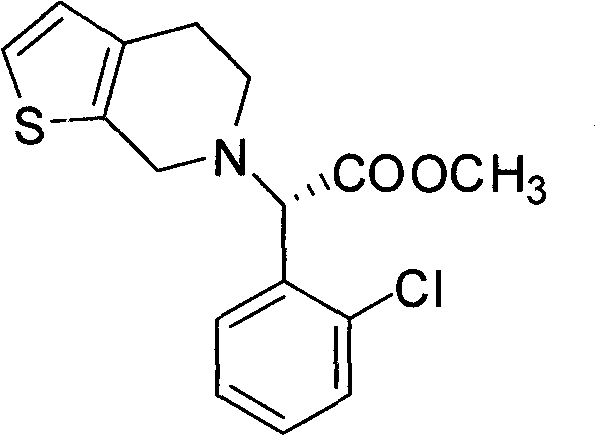
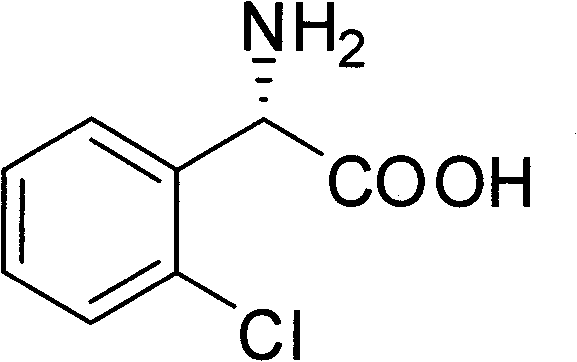
Chemical-enzyme method for preparing (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate
A technology of o-chlorophenylglycine methyl ester and o-chlorophenylglycine, which is applied in the chemical-enzymatic field of preparing the chiral intermediate of clopidogrel-o-chlorophenylglycine methyl ester, and can solve the problem of affecting the optical purity of the end product clopidogrel , optical purity is not high, to achieve the elimination of recrystallization steps, high optical activity, high racemization rate and racemation yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
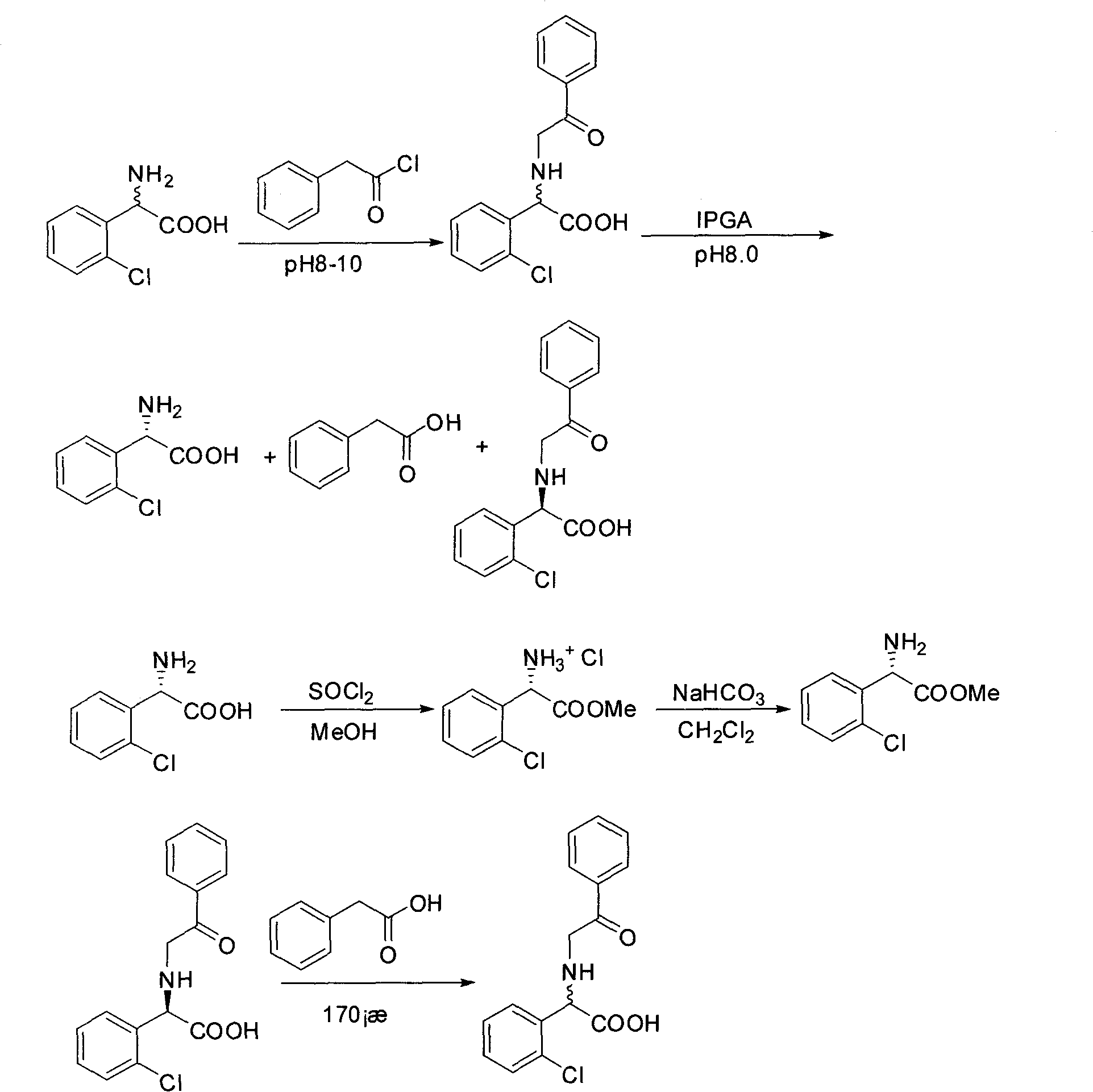
[0019] Embodiment 1: Preparation of (R, S)-N-phenylacetyl-o-chlorophenylglycine
[0020] Add 74.2g (0.4mol) (R, S)-o-chlorophenylglycine and 48g NaOH (1.2mol) into 700ml water, stir to dissolve. 64ml (0.48mol) of phenylacetyl chloride (or phenylacetic acid, or methyl phenylacetate, or phenylacetyl bromide) was added dropwise under ice-bath conditions. After the dropwise addition was completed, the reaction was carried out overnight at room temperature. Adjust the pH to 1-2 with hydrochloric acid, and precipitate (R, S)-N-phenylacetyl-o-chlorophenylglycine as a solid under stirring. Suction filtration and drying gave 115.6 g of (R, S)-N-phenylacetyl-o-chlorophenylglycine with a yield of 95%.
Embodiment 2
[0021] Example 2: (R, S)-N-phenylacetyl-o-chlorophenylglycine enzyme catalyzed hydrolysis
[0022] Add 91.2g (0.3mol) of (R,S)-N-phenylacetyl-o-chlorophenylglycine to 600ml of water, and adjust the pH to 8.0 with ammonia water. Add 18.3 g of immobilized penicillin acylase, and stir the reaction at 30° C. for 12 h. Remove immobilized penicillin acylase by suction filtration. The filtrate was adjusted to pH 1-2 with concentrated hydrochloric acid, filtered with suction, the solid was washed with hot water, and dried to obtain 44.4 g of (R)-N-phenylacetyl-o-chlorophenylglycine with a yield of 97%. The filtrate was concentrated under reduced pressure at 60°C, and the isoelectric point was adjusted to precipitate a solid. The solid was washed with absolute ethanol and dried to obtain 12.6 g of (S)-o-chlorophenylglycine with a yield of 90% and ee of 100%.
Embodiment 3
[0023] Embodiment 3: Preparation of (S)-o-chlorophenylglycine methyl ester hydrochloride
[0024] Add 18.6g (0.1mol) (S)-o-chlorophenylglycine to 200ml of anhydrous methanol, and add SOCl dropwise in an ice bath (0-5°C) 2 14.5ml. After the dropwise addition was completed, the reaction was carried out at room temperature for 5h. Evaporate to dryness under reduced pressure at 60°C, and dry in vacuo to obtain 23.1 g of (S)-o-chlorophenylglycine methyl ester hydrochloride, with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com