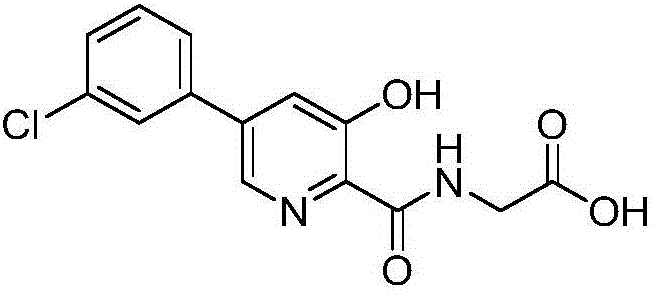
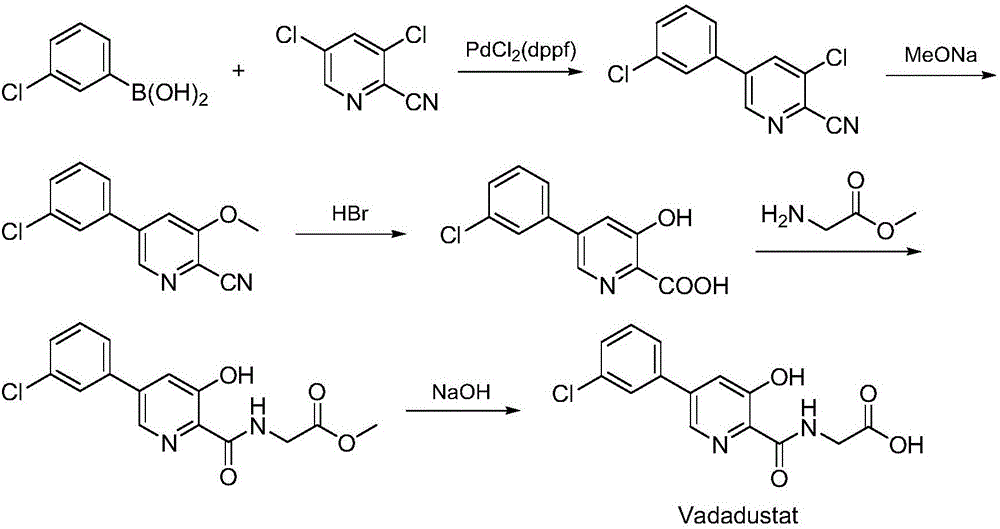
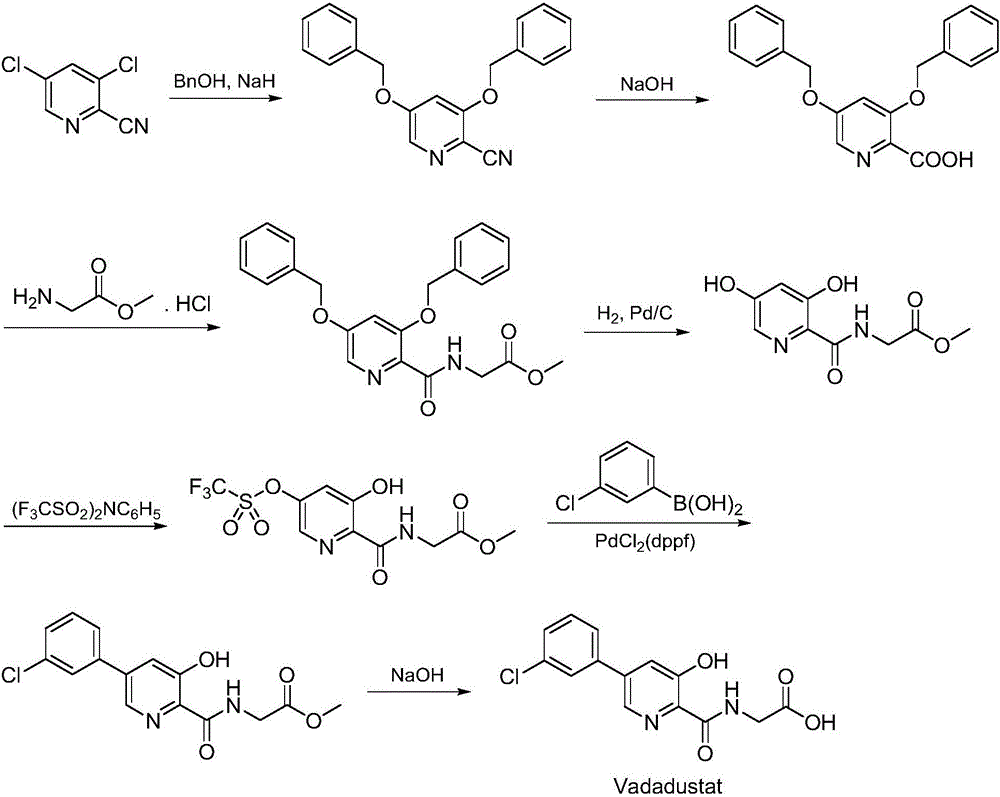
Synthesis method of Vadadustat
A synthesis method and condensing agent technology, applied in the direction of organic chemistry, etc., can solve the problems of short process flow, long synthesis steps, cumbersome operation, etc., and achieve the effect of reasonable technical scheme, simplified operation, and easy access to reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A) Preparation of N-(3,5-dichloropyridine-2-carbonyl) glycine methyl ester:
[0029] 3,5-dichloro-2-pyridinecarboxylic acid (19.2g, 0.10mol) and N,N'-carbonyldiimidazole (24.3g, 0.15mol) were dissolved in N,N-dimethylformamide (100mL), added Glycine methyl ester hydrochloride (15.1g, 0.12mol) was added dropwise with N,N-diisopropylethylamine (51.7g, 0.40mol), the reaction mixture was stirred at 35°C for 8 hours, and TLC was spotted to confirm that the reaction was complete. The reaction solution was concentrated to dryness by rotary evaporation, adjusted to neutrality by adding dilute hydrochloric acid, extracted by adding ethyl acetate, dried over magnesium sulfate, concentrated by rotary evaporation to dryness, and recrystallized from methanol to obtain N-(3,5-dichloropyridine-2- Carbonyl) glycine methyl ester, off-white solid (21.6g), yield 82.0%, the reaction formula of this step is as follows:
[0030]
[0031] B) Preparation of N-[5-(3-chlorophenyl)-3-chloropy...
Embodiment 2
[0041] A) Preparation of N-(3,5-dichloropyridine-2-carbonyl) glycine methyl ester:
[0042] 3,5-dichloro-2-pyridinecarboxylic acid (20.5g, 0.107mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (26.5g, 0.17mol) were dissolved in N , N-dimethylacetamide (130mL), added glycine methyl ester hydrochloride (17.4g, 0.139mol), added dropwise triethylamine (54.0g, 0.53mol), the reaction mixture was stirred at 25°C for 10 hours, TLC Spot the plate to confirm the completion of the reaction, the reaction solution was concentrated to dryness by rotary evaporation, adjusted to neutrality by adding dilute hydrochloric acid, extracted by adding ethyl acetate, dried over magnesium sulfate, concentrated by rotary evaporation to dryness, and recrystallized from methanol to obtain N-(3,5- Dichloropyridine-2-carbonyl) glycine methyl ester, off-white solid (24.0g), yield 85.4%, the reaction formula of this step is the same as Example 1;
[0043] B) Preparation of N-[5-(3-chlorophenyl)-3-chlo...
Embodiment 3
[0050] A) Preparation of N-(3,5-dichloropyridine-2-carbonyl) glycine methyl ester:
[0051] 3,5-Dichloro-2-pyridinecarboxylic acid (30.0g, 0.16mol) and 1-hydroxybenzotriazole (35.9g, 0.27mol) were dissolved in N,N-dimethylformamide (220mL), added Glycine methyl ester hydrochloride (26.6g, 0.21mol), 4-dimethylaminopyridine (105.0g, 0.86mol) was added dropwise, and the reaction mixture was stirred and reacted at 30°C for 12 hours. TLC spotting confirmed that the reaction was complete, and the reaction solution was rotary evaporated Concentrate to dryness, add dilute hydrochloric acid to adjust to neutrality, add ethyl acetate to extract, dry over magnesium sulfate, concentrate to dryness by rotary evaporation, and recrystallize from methanol to obtain N-(3,5-dichloropyridine-2-carbonyl)glycine methyl Ester, off-white solid (33.6g), yield 81.7%, the reaction formula of this step is the same as Example 1;
[0052] B) Preparation of N-[5-(3-chlorophenyl)-3-chloropyridine-2-carbony...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com